
Influenza in Sweden – Season 2020–2021
Summary
There was no influenza epidemic in Sweden during the 2020-2021 influenza surveillance season, due to a combination of factors stemming from the current COVID-19 pandemic. Measures to reduce the spread of COVID-19 both nationally and globally contributed to a reduction in the transmission of influenza, while a sharp reduction in travel has also meant that influenza was only sporadically introduced to Sweden and the rest of Europe from countries with ongoing transmission. The spread of influenza has been very low in the northern hemisphere and in most of the rest of the world. Only 29 sporadic cases of laboratory-confirmed influenza were reported in Sweden. The number of cases reported nationally stayed below the threshold value for epidemic start throughout the surveillance period from week 40, 2020, until week 20, 2021. The number of reported cases was unusually low despite high testing, and the number of analysed samples (approximately 175,000) was higher in comparison to the average number of samples analysed during the last four seasons (79,000).
Of the 29 cases reported, 19 cases were influenza B and 10 were influenza A. Of the influenza B cases, six were typed for lineage of which all were influenza B/Victoria. The seven subtyped influenza A samples were exclusively influenza A(H3N2). More men (59 percent) than women (41 percent) were reported with influenza, but the difference was not statistically significant. Web searches and telephone calls to the medical advice line 1177 reflected exceptionally low levels of influenza activity during the season.
During the 2020–2021 season, one patient with influenza was reported having received intensive care. The patient was diagnosed with influenza B/Victoria and had a medical risk factor for severe influenza illness. Because there was no influenza activity during the season, no influenza-related mortality was measured.
Influenza was not detected in any of the 543 samples analysed in sentinel surveillance of patients presenting with influenza-like illness (ILI) or acute respiratory illness (ARI) in primary care facilities. The Public Health Agency participates in the European Influenza Monitoring Vaccine Effectiveness (I-MOVE) network with data from Swedish sentinel sampling. Due to the very low number of influenza cases this season, no vaccine effectiveness could be estimated.
The influenza vaccination campaign began in week 45 (first week of November). Based on the ongoing pandemic and the expected increased demand for influenza vaccination, the Public Health Agency and the County Medical Officers decided that risk groups and healthcare workers would be prioritized for influenza vaccination during November. During the 2020–2021 season, the average vaccination coverage among people 65 years and older was 60 percent, which is seven percentage points higher than the previous season. In total, over 1,250,000 people aged 65 years and older are estimated to have been vaccinated against influenza.
Virus characterisation is normally performed on a selection of the influenza-positive samples from the sentinel surveillance programme and from laboratories. For the present season, however, because the number of influenza-positive samples was low all received samples containing sufficient virus for sequencing were genetically characterised. In total, 11 viruses were genetically characterised by whole genome sequencing, of which six were influenza A(H3N2) and five were B/Victoria. These were all shown to belong to genetic subgroups that, in combination with the additionally detected sets of amino acid substitutions, have shown reduced antigenic similarity to the respective vaccine viruses that were recommended for the northern hemisphere season 2020–2021. No mutations inducing reduced susceptibility to neuraminidase inhibitors were determined in any of these 11 viruses. Seven of the viruses were also analysed for phenotypic susceptibility to neuraminidase inhibitors, and all were susceptible.
Sammanfattning
Influensaepidemin uteblev under säsongen 2020–2021 i Sverige, på grund av en kombination av faktorer som rör den rådande covid-19 pandemin. Åtgärder för att minska spridningen av covid-19 både nationellt och globalt bidrog till minskad smittspridning av influensa. Dessutom har ett kraftigt minskat resande också betytt att influensa endast sporadiskt introducerades till Sverige och övriga Europa från länder med pågående spridning. Spridningen av influensa var mycket låg på norra halvklotet och i stora delar av övriga världen. Endast 29 sporadiska fall av laboratoriebekräftad influensa rapporterades i Sverige. Antalet fall i landet höll sig under tröskelvärdet för epidemistart under hela övervakningsperioden, från vecka 40 2020 till vecka 20 2021. Antalet fall var ovanligt lågt trots att fler prover än vanligt analyserades för influensa: cirka 175 000 prover, jämfört med i genomsnitt cirka 79 000 prover under de föregående fyra säsongerna.
Av de 29 rapporterade fallen var 19 influensa B och 10 influensa A. Av influensa B-fallen linjetypades 6 fall och samtliga var B/Victoria. Samtliga 7 subtypade influensa A-prover var influensa A(H3N2). Fler män (59 procent) än kvinnor (41 procent) fick laboratoriebekräftad influensa men skillnaden var inte statistiskt signifikant. Både Webbsök för influensa och statistik över kontaktorsaker till 1177 Vårdguiden på telefon visade en mycket låg influensaaktivitet under säsongen.
Under säsongen 2020–2021 har en patient med laboratorieverifierad influensa rapporterats som vårdad på intensivvårdsavdelning. Patienten hade influensa B/Victoria och tillhörde en riskgrupp för svår influensasjukdom. Eftersom influensa inte spreds i Sverige under säsongen finns ingen influensarelaterad effekt på mortaliteten.
Inom sentinelprovtagning kunde influensa inte påvisas i något av 543 analyserade prover. Folkhälsomyndigheten deltar i det europeiska nätverket I-MOVE för att mäta influensavaccinets effekt med data från den svenska sentinelprovtagningen. På grund av mycket låg influensaaktivitet fanns inte tillräcklig data för analys av vaccinationseffekt för 2020–2021.
Höstens vaccinationskampanjer mot säsongsinfluensa startade vecka 45 (första veckan i november). Utifrån den rådande pandemin förväntades ökad efterfrågan, och därför beslöt Folkhälsomyndigheten och landets smittskyddsläkare att personer i riskgrupper och vård- och omsorgspersonal skulle prioriteras för vaccination. Under säsongen 2020–2021 var vaccinationstäckningen 60 procent bland personer 65 år och äldre, vilket är sju procentenheter högre än föregående säsong. Uppskattningsvis var det drygt 1 250 000 personer i åldersgruppen 65 år och äldre som vaccinerade sig.
Viruskaraktärisering görs i normala fall på ett urval av de stammar som samlas in genom sentinelprovtagningen och från laboratorier i landet. Under säsongen var dock de influensapositiva proverna så få att samtliga prover med tillräckligt hög viruskoncentration genomgick vidare karaktärisering med sekvensering. Totalt genomgick 11 stammar genetisk karaktärisering, varav 6 A(H3N2)-stammar och 5 B/Victoria-stammar. Samtliga stammar visade sig tillhöra genetiska subgrupper som i kombination med ett antal ytterligare påvisade aminosyrautbyten uppvisar lägre antigenisk likhet till de rekommenderade vaccinstammarna för säsongen 2020–2021. Ingen av de 11 stammarna bar på något av de aminosyrautbyten som är kända för att ge nedsatt känslighet för neuraminidashämmare. Dessutom analyserades 7 av stammarna för fenotypisk känslighet för neuraminidashämmare och samtliga var känsliga.
About the publication
This report describes the monitoring systems for influenza in use during the winter season of 2020–2021 and the results of both epidemiological and virological surveillance. Data are also compared to previous influenza seasons.
The report has been prepared for the World Health Organization (WHO) as part of the Public Health Agency of Sweden’s function as a National Influenza Centre (NIC).
Annual reports in English about the influenza seasons in Sweden have been available since 1997, and those from 2000–2001 onward can be found on the Public Health Agency’s website (suggested search “Influenza in Sweden”) (1).
Public Health Agency of Sweden
Mia Brytting
Head of Unit
Unit for Laboratory Surveillance of Viral
Pathogens and Vaccine Preventable Diseases
Anneli Carlander
Head of Unit
Unit for Coordination and Surveillance COVID-19
Influenza surveillance in Sweden
Influenza epidemics recur in Sweden each winter, with a range of effects depending on the characteristics of the circulating viruses and the level of immunity in different age groups. New influenza strains, particularly those different enough to cause a pandemic, can cause a high number of severe cases, which can cause great strain on intensive care units and can lead to deaths in all age groups. None of these effects are detectable through a single reporting system. In order to get an overall picture of on-going influenza activity and to remain prepared in case of an influenza pandemic, the Public Health Agency of Sweden (Folkhälsomyndigheten) has a number of different epidemiological reporting systems for influenza ranging from the collection of data from different healthcare providers to the analysis of web searches.
Virological surveillance is as important as epidemiological reporting systems. Viruses are typed as influenza A or B by regional laboratories in real time during the influenza season, and some laboratories also determine the subtype for influenza A. Throughout the season, viruses from around the country are characterised by the Public Health Agency with regard to subtype and lineage, vaccine similarity, sensitivity to antiviral drugs, and other factors that might affect the severity of the infections they cause. Viruses are also isolated and sent to the WHO Collaborating Centre (WHO CC) in London for further characterisation and to provide a basis for vaccine strain selection. When new strains of influenza virus emerge, reference methods for diagnostics are established at the Public Health Agency and shared with all microbiological laboratories in Sweden.
During the influenza season, the Public Health Agency condenses national and international data into a detailed weekly bulletin that is published on the agency’s website (2). A preliminary summary of the 2020–2021 season was published on June 10, 2021 (3). The bulletin provides timely analysis of the current situation in Sweden and abroad and has a wide readership.
Effects of the COVID-19 pandemic
The COVID-19 pandemic had a significant impact on the surveillance and transmission of influenza in Sweden during the 2020-2021 influenza surveillance season. Sweden saw changes to healthcare seeking and testing behaviour and increased calls to medical advice lines. Measures to reduce the spread of COVID-19 both nationally and globally contributed to a reduction in the transmission of influenza in most countries of the Northern hemisphere to a very low level during the season, as in most of the rest of the world. In Sweden, measures included social distancing, testing and tracing of cases, travel restrictions, changes in sick leave policies and advice to stay home if symptomatic, advice to work from home if possible, distance learning for high schools and universities, restrictions in opening hours and capacity of restaurants and bars, and more. Globally, very low influenza activity was reported by WHO (4). A sharp reduction in travel meant that influenza was only sporadically introduced to Sweden and the rest of Europe from countries with ongoing transmission.
Updates to national policies and recommendations
During the 2020–2021 season, updated web-based information about influenza vaccination and surveillance, as well as about influenza and COVID-19, were published continuously throughout the season.
Updated vaccination recommendations were published in September 2020, including the addition of healthcare personnel to recommended groups
Recommendations for influenza vaccination of risk groups (5).
In addition, a short summary document regarding newer influenza vaccines was published in March 2021:
Report: New Seasonal Influenza Vaccines, March 2021 (6)
The COVID-19 pandemic changed both practical planning and communication for seasonal influenza vaccination compared to previous seasons. The Public Health Agency was commissioned by the government to strengthen the information given to risk groups about seasonal influenza vaccination during the autumn of 2020, with a special focus on reaching groups and areas where vaccination coverage is lower.
Surveillance 2020–2021
The pyramid below illustrates the different outcomes and levels of severity and the corresponding surveillance systems for individuals infected with influenza (Figure 1), ranging from the large number of infected individuals to the small number who die as a result of the influenza infection. The data collection systems that the Public Health Agency used to monitor influenza activity at the various levels of the influenza pyramid in Sweden during the 2020–2021 season are described in the sections of this report, along with the results of each system. Each system is described further at the start of each section below showing the season’s results in detail. The COVID-19 pandemic had a significant impact on influenza surveillance throughout the 2020-2021 season, with changes to healthcare seeking and testing behaviour and increased calls to medical advice lines.
Figure 1. The “influenza pyramid” showing possible outcomes of an influenza infection and the surveillance systems at each level.
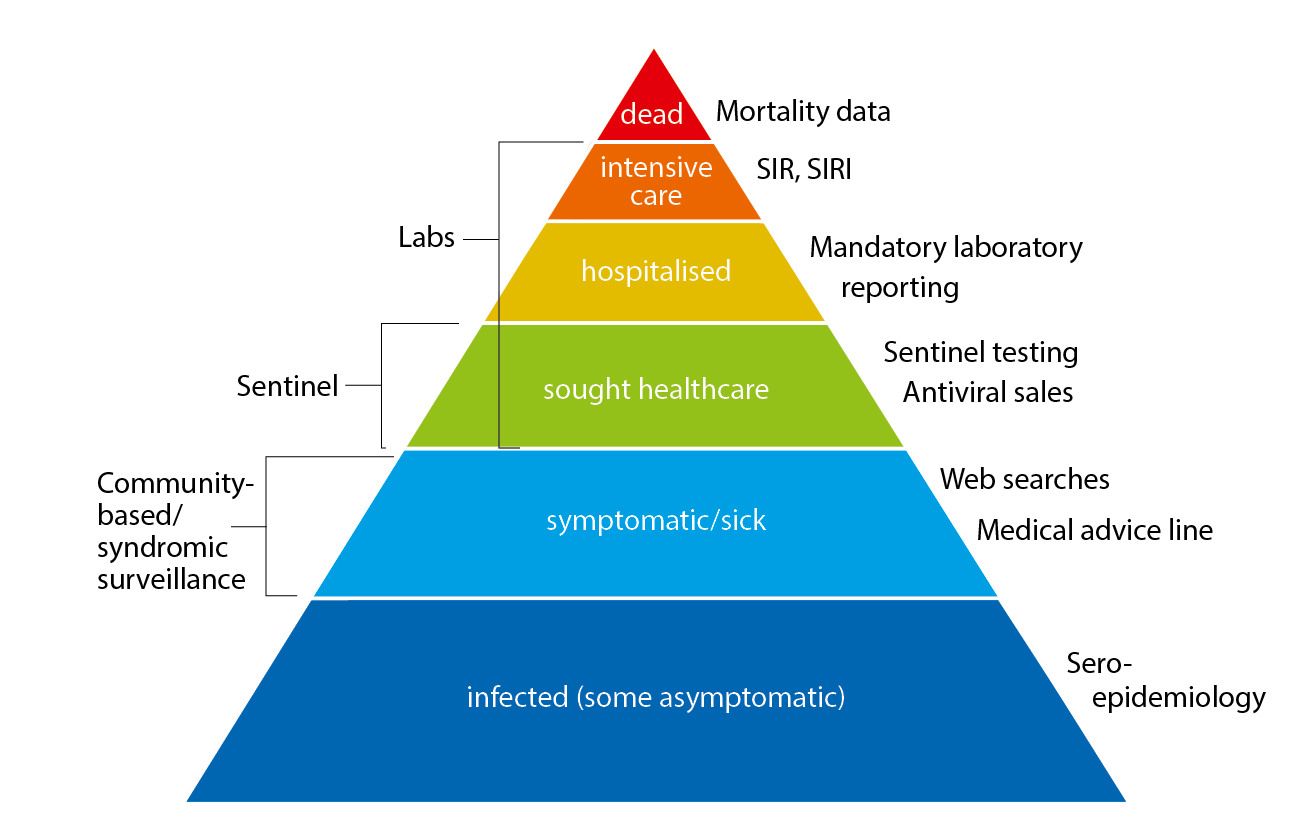
SIR: Swedish Intensive Care Registry. SIRI: Swedish Intensive Care Registry – Influenza module
Epidemiological Analyses
Laboratory-based surveillance
All laboratory-confirmed cases of influenza have statutory reporting requirements (as of Dec 1, 2015), but subtyping is not required. Denominator data (the total number of samples analysed) are reported voluntarily via e-mail. Samples analysed for influenza at the laboratories in Sweden have historically primarily been done in hospital settings, but the proportion of cases reported from outpatient settings has increased in recent years.
The 2020–2021 influenza season was unusually mild, and only 29 sporadic cases of influenza were reported (see Figure 2). The number of cases stayed below the threshold value for epidemic start throughout the period from week 40, 2020, until week 20, 2021. Due to the ongoing COVID-19 pandemic and restrictions implemented both in Sweden and elsewhere, the spread of influenza virus has drastically decreased.
Of the 29 cases reported, 19 cases were influenza B and 10 were influenza A. Of the influenza B cases, six were typed for lineage and all were influenza B/Victoria. Of the seven subtyped influenza A cases, all were influenza A(H3N2).
The median age of cases should be interpreted with caution because the number of reported influenza cases was low overall and by type. The overall median age for laboratory-confirmed influenza was 35 years. For influenza A and B the median age was 32 years and 54 years, respectively (see Table 1). More men (59 percent) than women (41 percent) were reported with influenza, but the difference was not statistically significant.
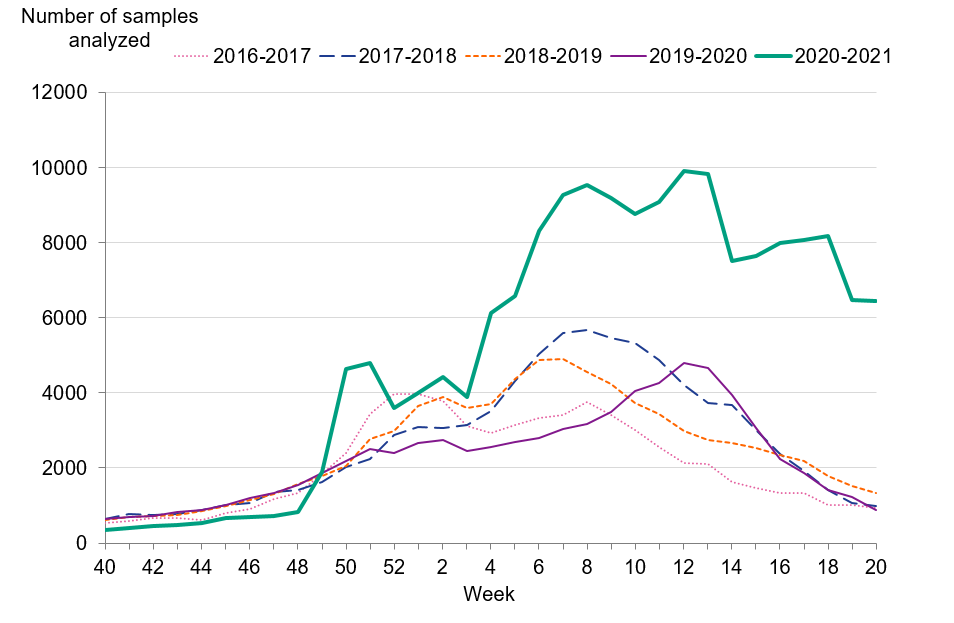
Although the number of reported cases was unusually low, the number of analysed samples (approximately 175,000) was higher in comparison to the average number of samples analysed during the last four seasons (79,000). Figure 3 shows the number of samples analysed per week and season. Table 2 summarises the laboratory reporting results over the last six seasons, including the number of analysed samples and the proportion of positive samples as well as the total.
| Indicator | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 |
|---|---|---|---|---|---|
| Dominant type | A(H3N2) | B/Yamagata | A(H1N1)pdm09 | Mixed season | No season |
| Median age influenza A | 73 | 70 | 60 | 51 | 32 (a) |
| Median age influenza B | 58 | 68 | 37 | 24 | 54 (a) |
(a) The median age should be interpreted with caution because the number of reported influenza cases was low.
Figure 2. Total number of laboratory-confirmed cases of influenza (all types) per week and the dominating influenza type(s) per season, 2016–2017 to 2020–2021.
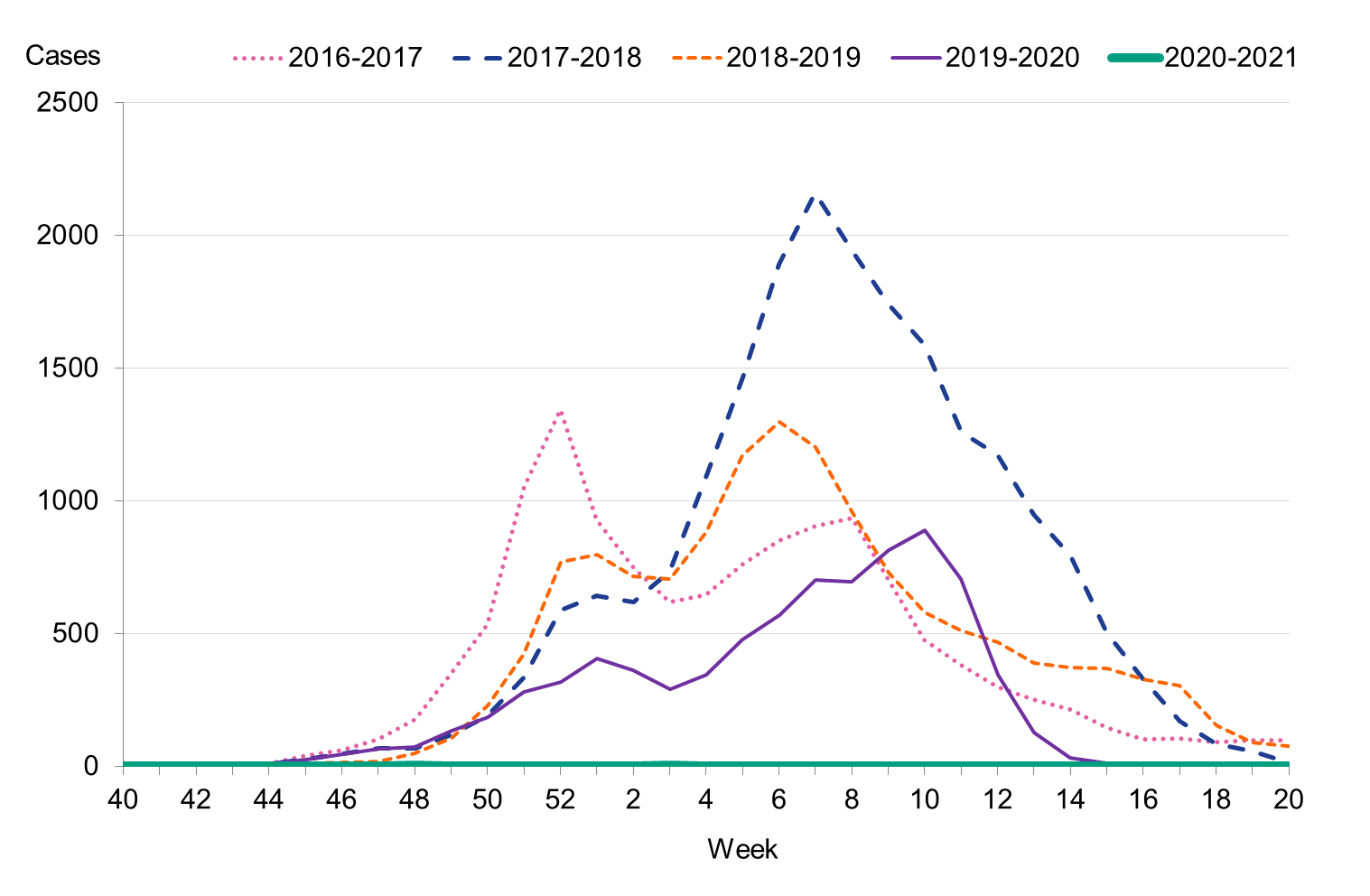
Figure 3. Number of samples analysed per week, 2016–2017 to 2020–2021.
| Indicator | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 |
|---|---|---|---|---|---|
| Analysed samples | 68,241 | 88,837 | 83,325 | 75,819 | 175,048 |
| Proportion positive samples (percent) | 19 | 23 | 17 | 10 | 0,02 |
| Total positive for influenza A | 12,361 | 7,406 | 13,664 | 5,441 | 10 |
| Total positive for influenza B | 708 | 13,280 | 93 | 2,500 | 19 |
Antiviral sales
Every Monday, the Public Health Agency receives data from the Swedish eHealth Agency on the previous week’s sales of the antivirals zanamivir and oseltamivir. Data include all sales categories, i.e. prescriptions and healthcare requisitions. The number of prescriptions directly reflects patients seeking medical care for influenza-like symptoms, whereas requisitions more likely show medical staff preparations for intense periods as well as reflect patients currently in treatment.
Cumulative sales of antiviral medications were at much lower levels compared to the last four seasons (see Table 3). During the 2020–2021 season, requisitions reached their highest level during week 48, 2020, despite the fact that the influenza cases were still at very low levels, and this was likely in preparation for a possible increase in influenza cases.
| Indicator | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 |
|---|---|---|---|---|---|
| Prescriptions | 4,782 | 7,120 | 6,086 | 5,263 | 774 |
| Health care requisitions | 10,144 | 12,790 | 10,534 | 7,252 | 1,370 |
| Total sales | 14,926 | 19,910 | 16,620 | 12,515 | 2,144 |
| Total influenza cases | 13,069 | 20,686 | 13,757 | 7,941 | 29 |
Influenza cases in intensive care
In collaboration with Swedish Intensive Care Registry (SIR), the Public Health Agency receives data daily on influenza and COVID-19 patients in intensive care. Data are reported by the intensive care units through a special module in the registry, known as SIRI. The case-based reporting of influenza patients in the intensive care is the basis of severe disease surveillance and allows for the early detection of increases in the number of severe influenza cases.
During the 2020–2021 season, one patient with influenza was reported as having received intensive care. The patient was diagnosed with influenza B/Victoria and had a medical risk factor for severe influenza illness.
Influenza-related mortality
Because there was no influenza activity during the season, no influenza-related excess mortality was measured. Analysis of deaths among laboratory-confirmed influenza patients was not completed due to the low number of cases.
Virological analyses
Sentinel sampling
Virological analysis of samples from sentinel general practitioners, infectious disease clinics, and paediatric clinics contributes to national and international surveillance of circulating influenza viruses. In order to estimate what proportion of the patients seeking care for influenza-like illness (ILI), as well as some patients with acute respiratory illness (ARI), actually has influenza, the different clinics are encouraged to collect nasal samples from patients with ILI. Patient characteristics, including age, sex, risk factors, syndrome (ILI vs. ARI), and vaccination status, are analysed with respect to the types of influenza that are circulating. The Public Health Agency carries out laboratory analyses for influenza free of charge for these samples. Representative positive samples are also used to characterise the circulating strains of influenza by sequencing and by antiviral susceptibility testing.
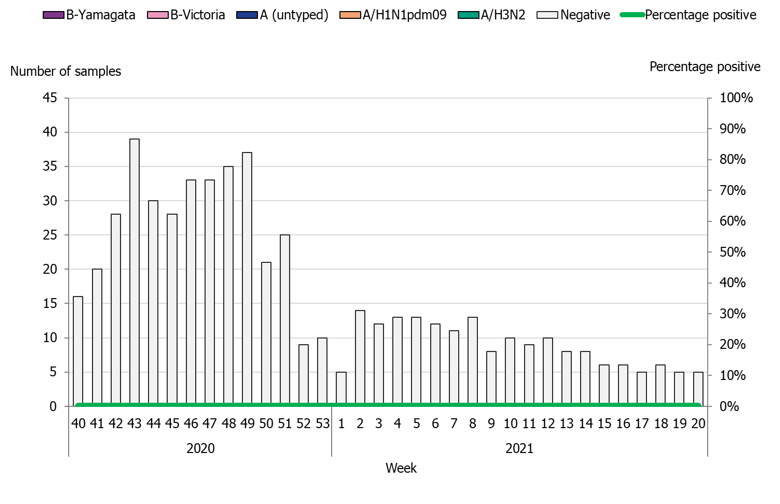
During the 2020–2021 season, 543 sentinel samples were submitted from 72 sentinel sampling sites, including 69 general practitioners and 3 paediatric or infectious disease clinics. Eighty-nine percent of the samples were collected by general practitioners. In total, zero samples tested positive for influenza. Figure 4 shows the distribution of samples taken. As of week 10, 2020, analysis for SARS-CoV-2 is included in the analysis of sentinel samples. Sentinel sampling has periodically been included in the weekly reports for COVID-19 on our website (8).
The Public Health Agency participates in the European Influenza Monitoring Vaccine Effectiveness (I-MOVE) network with data from Swedish sentinel sampling. Due to the very low number of influenza cases this season, no vaccine effectiveness could be estimated, and a final report is pending.
Figure 4. Number of sentinel samples submitted each week, number of samples by subtype/lineage, and the percentage positive, 2020–2021.
Clinical features
Of the 543 patients sampled through the sentinel system, 77 percent had ILI, 19 percent had ARI, and 4 percent had no information on symptoms. In total, 57 percent (n = 310) of the patients were female with a median age of 44 years and 43 percent (n = 233) were male with a median age of 38 years. Twenty-two percent of the samples were collected from patients belonging to a risk group due to age and/or medical condition. Table 4 summarises the laboratory results from the sentinel sampling system for the last three seasons, including the number of samples.
Vaccination status was reported for 480 (88 percent) of the 543 patients sampled during the season. Of these, 40 (7 percent) were vaccinated. Among the patients belonging to a risk group, 26 percent were vaccinated.
| Samples | 2018–2019 | 2019–2020 | 2020–2021 |
|---|---|---|---|
| Analysed | 1,323 | 2,102 | 543 |
| Negative | 915 | 1,718 | 543 |
| Positive | 408 | 384 | 0 |
| Proportion positive (percent) | 31 | 18 | 0 |
| Positive for influenza A | 403 | 218 | 0 |
| Positive for influenza B | 5 | 166 | 0 |
Subtyping and lineage determination
All diagnostic laboratories perform influenza typing using molecular assays for influenza A and B. During the 2020–2021 season, subtyping was performed at two regional laboratories (Lund and Gothenburg). The Public Health Agency performs subtyping and lineage typing by real-time PCR for all samples sent in from the diagnostic laboratories and on all influenza-positive samples from sentinel surveillance.
In total, seven influenza A-positive samples from laboratories in Sweden were subtyped during the season, of which all were influenza A(H3N2).
The lineage was determined for six influenza B-positive samples, of which all belonged to the B/Victoria lineage. Typing results for subtype and lineage from sentinel and laboratory reporting systems are presented in Table 5.
| Season and system | A(H1N1)pdm09 | A(H3N2) | B/Victoria | B/Yamagata |
|---|---|---|---|---|
| 2016–2017 Sent. (%) | 1 | 96 | 1 | 2 |
| 2016–2017 Lab (%) | <1 | >99 | <1 | <1 |
| 2017–2018 Sent. (%) | 6 | 17 | <1 | 77 |
| 2017–2018 Lab (%) | 13 | 23 | <1 | 64 |
| 2018–2019 Sent. (%) | 76 | 23 | 1 | <1 |
| 2018–2019 Lab (%) | 63 | 37 | <1 | <1 |
| 2019–2020 Sent. (%) | 33 | 23 | 44 | 0 |
| 2019–2020 Lab (%) | 25 | 44 | 31 | 0 |
| 2020–2021 Sent. (%) | 0 | 0 | 0 | 0 |
| 2020–2021 Lab (a) (%) | 0 | 34 | 66 | 0 |
(a) Data should be interpreted with caution as very few samples were available for determination of subtype/lineage.
Characterisation
A selection of the influenza-positive samples from laboratories and from the sentinel surveillance programme are further analysed for genotypic features by sequencing and for phenotypic sensitivity to neuraminidase (NA) inhibitors. Normally, samples are selected based on geographical location, period of collection, and types/subtypes/lineage types to achieve representativity. The Swedish laboratories are also asked to send influenza-positive samples from severely ill or deceased patients, patients with vaccine break-through infections, and patients who do not respond to antiviral treatment. However, because the number of influenza-positive samples was low this season, all viruses with a Ct-value of 33 or lower were selected for further genetic characterisation.
The main sequencing technology used is NGS (Next Generation Sequencing), which allows for whole-genome sequencing of all known influenza A subtypes and both influenza B lineage types. The hemagglutinin (HA) gene is characterised with respect to genetic vaccine similarity, clade affiliation, and changes in receptor affinity (lung receptors versus upper respiratory tract receptors). In addition, the HA target sequences for the subtype/lineage-specific real-time PCR systems used for detection of influenza in clinical samples are analysed for sequence mismatches compared to the real-time PCR primers and probes. The NA gene is analysed with respect to reduced or highly reduced inhibition by NA inhibitors. In addition to NGS, Sanger sequencing of the NA gene is used for more urgent requests of genotypic antiviral resistance testing.
Two aspects of the matrix protein (M gene) are analysed: the M2 gene of influenza A is analysed with respect to amantadine resistance and the M gene of influenza A and B is analysed for mismatches to the primers and probes of the real time PCR detection system.
Phenotypic analysis of sensitivity to the NA inhibitors oseltamivir (Tamiflu®/Ebilfumin®) and zanamivir (Relenza®) is performed with the NA inhibition (NAI) assay, which requires cell-propagated virus. A representative selection of the isolated virus samples are sent to the WHO CC in London for antigenic characterisation and for phenotypic analysis of sensitivity to NA inhibitors using the NAI assay.
All characterisation data are reported to TESSy and to the Global Initiative on Sharing All Influenza Data (GISAID).
Genetic groups
The genetic group of six characterised influenza A(H3N2) and five B/Victoria viruses from the 2020–2021 season are shown in Tables 6A-B and by influenza subtype or lineage and in the phylogenetic trees in Appendices 1–2. According to the report published by the WHO in conjunction with the influenza vaccine composition meeting for the northern hemisphere in February 2021, influenza A(H3N2) viruses in genetic subgroup 3C.2a1b+T131K-A, and having the additional amino acid substitutions Y159N, T160I, L164Q, G186D, and D190N, show reduced antigenic similarity to both the cell-based and egg-based vaccine viruses that were recommended for the northern hemisphere in the 2020–2021 season (10).
In addition, B/Victoria viruses in genetic subgroup 1Adel162-164B having the N150K, G184E, N197D, and R279K substitutions show reduced antigenic similarity to both the cell-based and egg-based vaccine viruses that were recommended for the northern hemisphere in the 2020–2021 season (10). Of the five characterised B/Victoria viruses, four had the additional amino acid substitutions N150K, G184E, N197D, and R279K, and one virus had N150K, G184E, N197E, and R279K. No influenza A(H1N1)pdm09 or B/Yamagata-positive samples were available for further characterisation this season.
| Genetic group/subgroup | Number of viruses | Percentage (%) |
|---|---|---|
| 3C.2a1b+T131K-A | 6 | 100 |
| 3C.2a1b+T131K-B | 0 | 0 |
| 3C.2a1b+T135K-A | 0 | 0 |
| 3C.2a1b+T135K-B (a) |
0 | 0 |
| 3C.3a | 0 | 0 |
(a) Genetic group of the vaccine virus recommended for the northern hemisphere in the 2020–2021 season (10).
| Genetic group/subgroup | Number | Percentage (%) |
|---|---|---|
| 1A |
0 | 0 |
| 1A del162-163 | 0 | 0 |
| 1A del162-164B (a) |
5 | 100 |
(a) Genetic group of the vaccine virus recommended for the northern hemisphere in the 2020–2021 season (10).
Antiviral sensitivity testing
The NA gene of six influenza A(H3N2) and five influenza B/Victoria viruses have been sequenced and analysed for amino acid substitutions known to confer reduced or highly reduced inhibition to the NA inhibitors oseltamivir and zanamivir. No such substitutions were detected this season.
Four of the A(H3N2) and three of the B/Victoria viruses were also analysed for phenotypic sensitivity to NA inhibitors (oseltamivir and zanamivir) by the Public Health Agency (six viruses) and WHO CC in London (one virus). All viruses were shown to be sensitive to both inhibitors.
The amantadine resistance mutation S31N was present in all six genetically characterised A(H3N2) viruses.
Virus isolation on cell culture
The majority of the samples selected for isolation are collected from other laboratories. The quality of the samples differs depending on, for example, the type of specimen, the time since sampling, and the storage and shipping conditions. Eleven of the collected samples with Ct ≤ 30 were cultured on MDCK-SIAT1 cells. One was not able to be cultured.
During the 2020–2021 season, 10 virus isolates and 1 clinical sample were shipped to the WHO CC London for further characterisation (one shipment in February and one in May).
Vaccination coverage
Data on vaccination coverage among persons 65 years of age and older were gathered by Sweden’s 21 county medical officers for their respective county councils starting in 2003. The Public Health Agency took over this task in 2014. Various methods for estimation have been used in different counties, including the use of vaccination registries, the number of vaccine doses given or distributed, sentinel reports on vaccination coverage, surveys among general practitioners, or patient record data. These methodological differences result in coverage estimates of varying quality and precision. Although the methods vary between counties, the methods within most counties have been roughly the same for the past several years, allowing a comparison over time. An estimate of the vaccination coverage in age groups younger than 65 is included, using data from a subset of county councils where registry data by age group are available throughout the season as well as annually. Data for 2020–2021 came from Gävleborg, Jämtland Härjedalen, Jönköping, Kalmar, Kronoberg, Norrbotten, Skåne, Stockholm, Sörmland, Värmland, Västernorrland, Västmanland, and Östergötland.
Coverage among persons 65 years of age and older
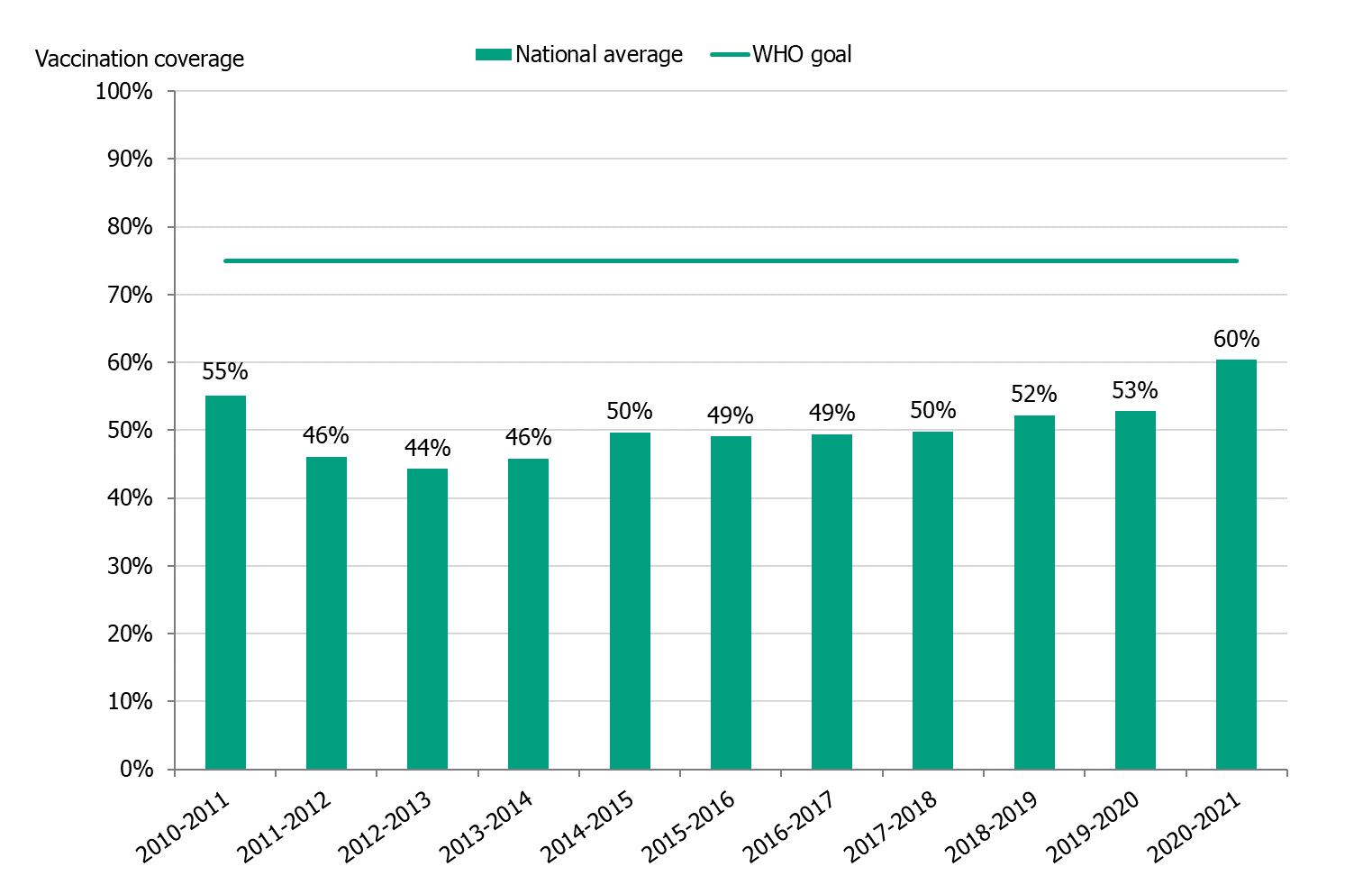
The national vaccination rate among persons aged 65 and over was 60 percent in 2020–2021, which was seven percentage points higher than the previous season (see Figure 5). The Public Health Agency estimates that over 1.25 million people aged 65 and older were vaccinated this season. Coverage was highest among people aged 75 to 84 years (64 percent), followed by those aged 85 and older (63 percent, see Table 7).
Figure 5. Vaccination coverage among persons aged 65 and older in Sweden, 2010–2011 to 2020–2021.
Regional differences in vaccination coverage
Comparisons among county councils are difficult because estimates use different methods. There is uncertainty associated with each value, but the figure below gives a picture of the current vaccination coverage rates for the age group 65 years and older throughout Sweden (see Figure 6). For more regional data and collection methods, see the Swedish-language summary of the season published in June 2021 (3). Percentages were calculated using the county population on December 31 of each year (Source: Statistics Sweden).
Figure 6. Estimated proportion of vaccinated persons aged 65 and older per county council in Sweden for the 2019–2020 and 2020–2021 seasons.
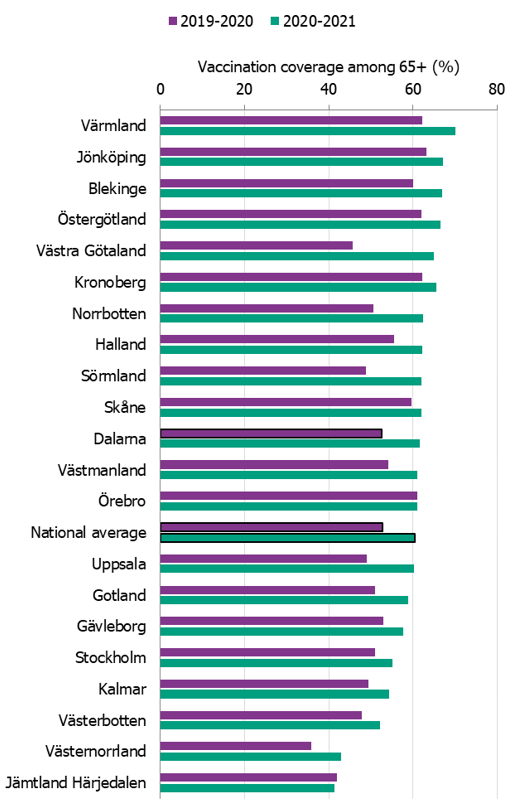
The data from Gotland come from the patient record system and directly from private healthcare offices. The data from Jämtland Härjedalen include vaccinations given within the regional healthcare system, and doses given at elder care and other facilities are under-reported, which means coverage is underestimated. For Skåne, there are two estimates of the coverage each season; 2019–2020: financial system (60%), vaccination register (49%); 2020–2021: financial system (62%), vaccination register (55%). All national analyses use the financial system estimates. Data from Stockholm only include vaccinations given to people in medical risk groups (including pregnancy) or those aged 65 years or older. Uppsala data are based on reports from public and private vaccination clinics through coding of risk groups as a basis for remuneration. Data from Västernorrland only include vaccinations given within the regional health care system, and doses given at elder care and other facilities are not included, which means the vaccination coverage is underestimated. Data from Västra Götaland for 2019–2020 come from vaccination registry data, which do not include vaccinations given at private vaccination clinics. For 2020–2021, the data come from a survey, which means the two seasons’ data are not comparable. In Örebro, coverage is calculated based on data from patient records, doses given at county care facilities, and data from private vaccination clinics, and doses given at hospitals are not included, which means coverage is underestimated. Data from Östergötland are based on patient records that include patients in county care facilities and the regional healthcare system, but not vaccinations given at private vaccination clinics, at pharmacies, or through occupational health services.
Vaccination coverage among persons under 65 years
It is difficult to estimate vaccination coverage among the medical risk groups under 65 years of age because these groups are hard to define and because data are often missing. Thirteen county councils (see note under Table 7) have reported the number of persons vaccinated under 65 years of age, although risk group status is often unknown. See Table 7 for a breakdown by age group. Please note, data from Östergötland is not included in the 2019–2020 data.
| Age group | 0–17 y | 18–39 y | 40–64 y | 65–74 y | 75–84 y | 85+ y |
|---|---|---|---|---|---|---|
| Percentage vaccinated 2019–2020 | 0.4% | 1.8% | 4.6% | 44.8% | 58.9% | 58.5% |
| Percentage vaccinated 2020–2010 | 0.4% | 2.2% | 6.2% | 52.3% | 64.1% | 62.8% |
| Population (31 Dec 2020) | 1,450,488 | 1,923,172 | 2,108,139 | 703,433 | 479,239 | 169,548 |
Recommended vaccine composition
In February 2021, the WHO recommended that the quadrivalentvaccines for use in the 2021–2022 northern hemisphere contain the following viruses (9).
Egg-based Vacciness
- an A/Victoria/2570/2019 (H1N1)pdm09-like virus;
- an A/Cambodia/e0826360/2020 (H3N2)-like virus;
- a B/Washington/02/2019 (B/Victoria lineage)-like virus; and
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
Cell- or recombinant-based Vaccines
- an A/Wisconsin/588/2019 (H1N1)pdm09-like virus;
- an A/Cambodia/e0826360/2020 (H3N2)-like virus;
- a B/Washington/02/2019 (B/Victoria lineage)-like virus; and
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
For trivalent vaccines, exclusion of the B/Phuket/3073/2013-like virus was recommended.
Syndromic surveillance
Two systems of syndromic surveillance were used during the 2020–2021 influenza season – Webbsök (Web search) and telephone calls to the medical advice line 1177 Vårdguiden. Webbsök is an automated system established in 2008 that uses a statistical model and completely anonymous data from a medical advice website to estimate the development of sentinel ILI incidence. Data are received daily and analysed weekly. The results are published on the web every Monday morning during the influenza season in the form of a graph, which is three days ahead of the publication of the weekly influenza bulletin.
In collaboration with the medical telephone advice line 1177 Vårdguiden, the Public Health Agency receives aggregated weekly data on calls from a system called Hälsoläge. The age group and main reason for calling are registered for all callers. The reported data include the number of calls related to cough, fever, and sore throat. The proportion of calls related to fever in children has been found to be a good indicator of influenza activity in the community.
Web search data (Webbsök)
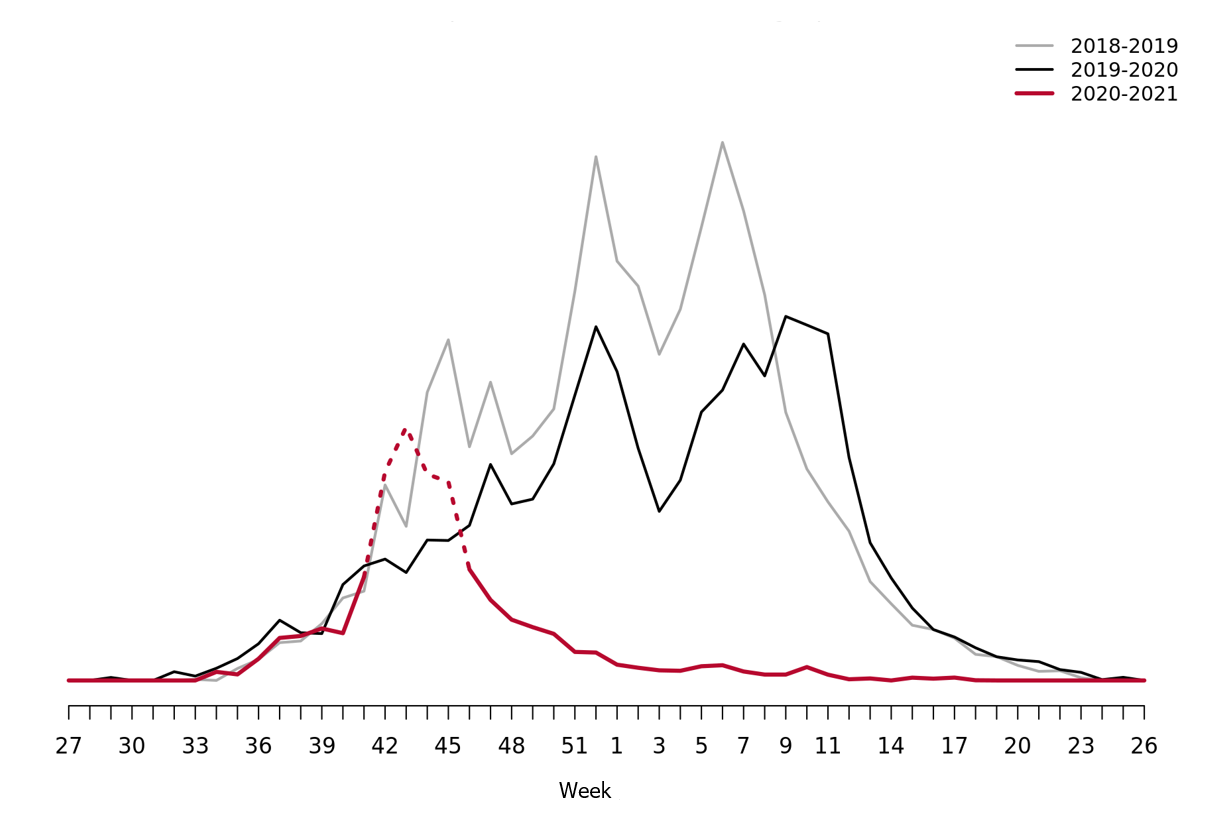
During the 2020-2021 influenza season the ILI-activity never reached the epidemic threshold. The influenza activity was at exceptionally low levels throughout the season (Figure 7).
Please note that the high levels during weeks 41–46 (dashed in the graph) corresponds to the influenza vaccination period, rather than increased influenza activity. The statistical model is sometimes affected by intensive media coverage and other factors that change how people search for information on 1177.se. Webbsök should therefore be interpreted with some caution and seen as a complement to the Public Health Agency’s traditional monitoring methods.
Figure 7. Webbsök’s estimated proportion of the population with ILI per week, 2018–2021.
Telephone advice line data (1177 Vårdguiden)
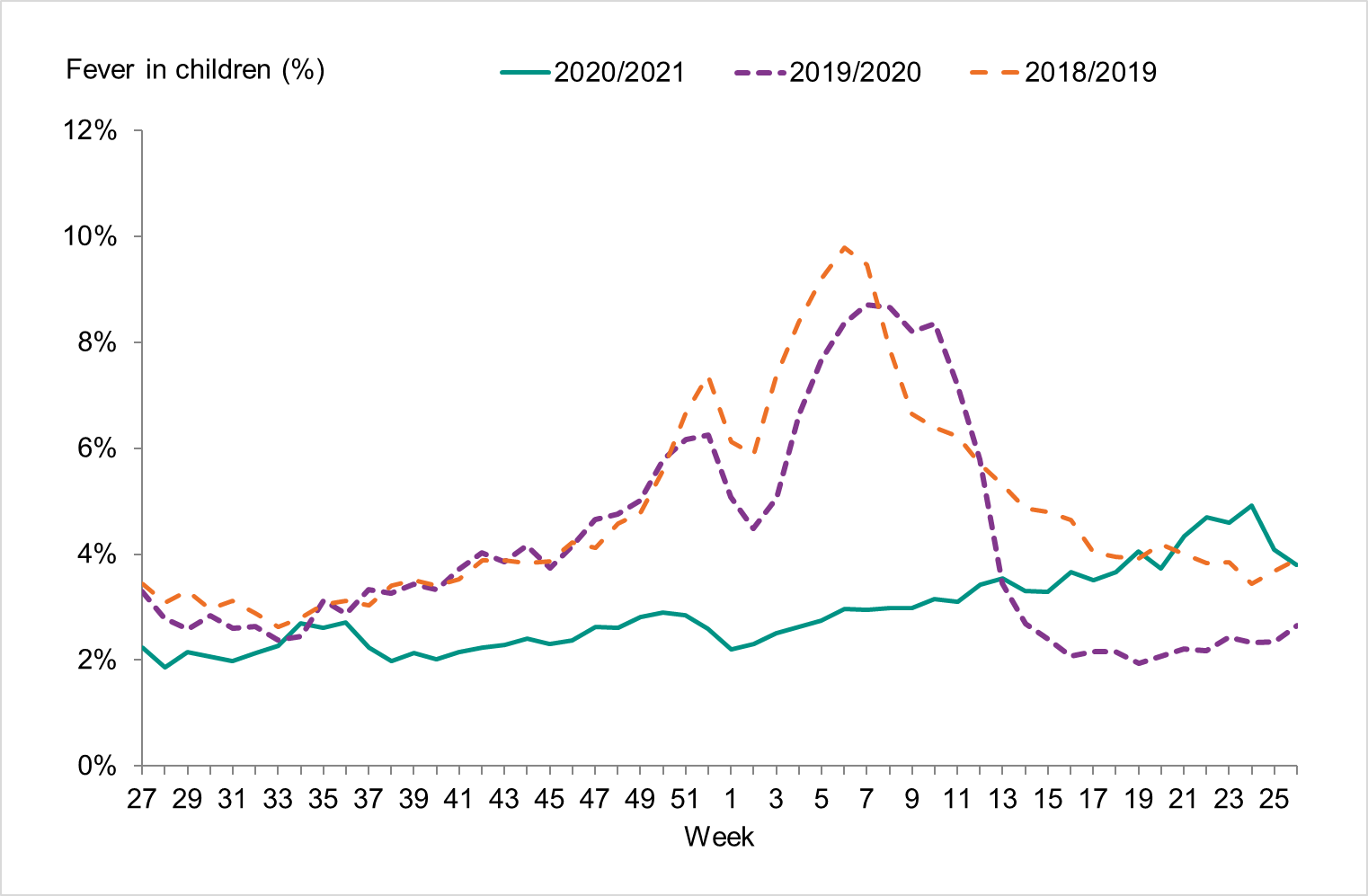
The number of calls to 1177 Vårdguiden regarding fever in children did not exceed the epidemic threshold during the 2020 2021 season. The percentage of calls regarding fever among children was at very low levels throughout the season.
Approximately 44,300 calls regarding fever in children were made to 1177 Vårdguiden, which accounted for 2.9% of all calls during the season (weeks 40–20) (Figure 8). This was much lower than the previous season when it was 5.0 percent. The highest number of calls (2,220) was registered during week 52, 2020. The highest percentage of calls (4.9 percent) were registered during week 24, 2021. Because there was no influenza circulation during the season, these data show the nonspecific nature of surveillance of fever among children, which can have many causes. Levels this season were considerably lower than the previous season peak (4,205 calls representing 8.7 percent of all calls).
Figure 8. Percentage of telephone calls regarding fever in children received by the medical advice line 1177 Vårdguiden for the past three seasons.
Quality assessment
Different external quality assessment programmes are used to ensure the quality of the diagnostics done in Sweden and of the analyses done by the Public Health Agency. Surveillance is dependent on standardised virological methods.
At the Public Health Agency, one-step real-time RT-PCR assays are used to detect influenza A and B, to subtype the influenza A-positive samples, and to discriminate between the two influenza B lineages. These assays have also been evaluated and implemented for avian influenza diagnostics. They are sensitive, rapid, and can easily be scaled up if necessary. The Public Health Agency continuously monitors the genomic sequences of circulating influenza strains to which the PCR assays are directed in order to detect mutations that could affect their sensitivity. The Public Health Agency also performs regular validation of each assay twice a year, both ahead of the influenza season and during the peak. The PCR protocols are shared with the other laboratories in Sweden, and the laboratories that use these PCR systems are encouraged to send all samples with deviating results to the agency for sequence analysis.
Every year the Public Health Agency produces a PCR-test panel for the Swedish laboratories on behalf of the External Quality Assessment for Clinical Laboratory Investigations (EQUALIS). This allows the laboratories to measure the analytic sensitivity and specificity of their method. The majority of the laboratories performing diagnostics for influenza use commercial rapid PCR kits. In the beginning of the season, a questionnaire is sent to all laboratories with a question concerning methods used. Kits used by the laboratories are selected for extra quality controls during the season in order to ensure that circulating influenza strains are detected with these assays. In the 2020–2021 season, aside from collecting information on the usage of rapid PCR systems, no samples were sent out to test these kits due to low circulation of influenza and prioritisations for COVID-19.
To ensure that the analyses performed at the Public Health Agency are correct, the agency participates in different external quality assessment programmes.
National quality assessment programme for influenza PCR
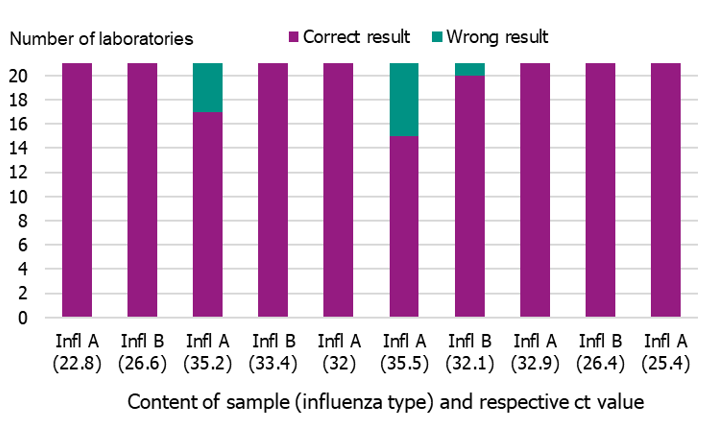
In September 2020, the Public Health Agency produced a new PCR panel for the Swedish laboratories, which was distributed by EQUALIS. Twenty-one laboratories participated in the panel, and 15 of these had all correct results (10/10). Two laboratories failed to detect one sample (9/19), three laboratories failed to detect two samples (8/10), and one laboratory failed to detect three samples (7/10) (Figure 9). Ct-values ranged from 22.8 to 39.9.
Figure 9. Results of the Swedish External Quality Assessment panel 2020.
Use of commercial rapid PCR-kits
During the 2020–2021 season, an increasing number of laboratories used commercial rapid PCR kits for influenza diagnostics. Results of a questionnaire sent to all 25 microbiological laboratories (24 replied) in Sweden showed that all laboratories used at least one commercial PCR assay, 11 used several commercial PCR assays, and seven laboratories used a combination of commercial and in-house assays.
The assays in use were:
- GeneXpert® Xpress Flu/RSV (Cepheid) (n = 16)
- FILMARRAY® Respiratory Panel (Biomérieux) (n = 9)
- In-House PCR (n = 7)
- Simplexa™ Influenza A/B & RSV Kit (Focus Diagnostics) (n = 5)
- ID Now™ (Alere™) Influenza A & B Test (Abbott) (n = 2)
- Allplex, panel 1, 2, and 3 (Seegene) (n = 2)
- VIASURE Flu A, Flu B & RSV RT PCR Kit (CerTest Biotec) (n = 1)
- ModularDx kit (TIB Molbiol) (n = 1)
The kits were not tested this season due to very low sample volumes and resource prioritisation for SARS-CoV-2 diagnostics.
External quality assessment programmes
The Public Health Agency participated in two external quality assessment (EQA) programmes during 2020. The results are summarised below.
European Influenza Surveillance Network EQA Programme
The Public Health Agency participated in the European External Influenza virus molecular detection (EISNINF_MD20), isolation (EISNINF_VI20), and antiviral susceptibility (EISNINF_AV20) EQA Programme through the European Influenza Surveillance Network (EISN).
Molecular detection
Molecular detection was successfully performed on all eight samples. All types
(4× INFL-A; 3× INFL-B) as well as all subtypes (3× A/H3, 1× A/H1pmd09) and lineages (2× B/Vic and 1x B/Yam) were correctly identified.
Virus isolation and characterisation
Virus isolation was performed by inoculation on MDCK-SIAT1 cells according to the SOPs of the WHO Collaborating Centre for Influenza Reference and Research, London. We reported results correctly as seven influenza-positive samples and one negative sample.
Genotypic characterisation based on the HA gene was performed by NGS (Ion Torrent), and the results were correct for all seven influenza-positive samples.
Antiviral susceptibility
Phenotypic analysis was performed using a MUNANA-based NAI assay. Eight of the nine influenza-positive samples were all identified correctly. One of the samples did not have enough NA activity for the assay, and sequencing revealed a large deletion not present in the NA sequence of the “original” sample (i.e. before isolation). This deletion was concluded to be the likely cause of the low NA activity seen in this isolate, which was in passage 2, as opposed to the eight other isolates which were all in lower passages.
Genotypic analysis of the NA gene was performed by NGS (Ion Torrent) and Sanger sequencing separately and was correctly reported for eight of the nine influenza-positive samples. For one of the A(H3N2)-samples having dual mutations (E119V+del245-248), the expected effect on the sensitivity to NA was interpreted (according to “Summary of neuraminidase amino acid substitutions associated with reduced inhibition by neuraminidase inhibitors NAI, Last updated April 2016) for each of these mutations individually as amino acid associated with highly reduced inhibition (AAHRI) for oseltamivir and amino acid associated with reduced inhibition (AARI) for zanamivir. A comment was also added that a synergistic effect resulting in higher level of resistance for zanamivir could not be excluded. The correct expected result for this sample was AAHRI for both oseltamivir and zanamivir. In the phenotypic analysis, highly reduced inhibition (HRI) for oseltamivir and for zanamivir was seen in this sample.
Annual WHO External Quality Assessment Panel for influenza
The Public Health Agency participated in the annual WHO External Quality Assessment Panel (EQAP) for influenza (no. 19) during 2020.
Molecular detection, typing, sub/lineage-typing
The 10 samples analysed by real-time PCR were correctly typed (A or B), and 9/10 were correctly subtyped. One sample (type A) could not be subtyped with this technique but was identified as subtype A(H9) by whole-genome sequencing (WGS) and later confirmed in the final report. This result was expected because the Public Health Agency does not have a real-time PCR system for H9 subtyping.
Genotypic and phenotypic antiviral susceptibility determination
Genotypic analysis of the NA gene was performed by NGS (Ion Torrent) and Sanger sequencing separately, and the results were correct for all three samples.
Phenotypic analysis was performed using a MUNANA-based NAI assay (fluorescence-based), and the results were correct for all three samples.
References
- Public Health Agency of Sweden. Publikationer [Publications]. [Internet]. Stockholm: Public Health Agency of Sweden; 2021 [cited 2021-08-25] Available from: http://www.folkhalsomyndigheten.se/publicerat-material/publikationer/
- Public Health Agency of Sweden, Aktuell influensarapport [Current Weekly Report]. [Internet]. Stockholm: Public Health Agency of Sweden; 2021 [cited 2021-08-25] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/influensa-veckorapporter/aktuell-influensarapport/
- Public Health Agency of Sweden, Sammanfattande rapport för säsongen 2020/2021 [Summary report for the 2020/2021 season]. [Internet] Stockholm: Public Health Agency of Sweden; 2020 [cited 2021-08-25] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/influensa-veckorapporter/arkiv-20202021/
- World Health Organization, Review of global influenza circulation, late 2019 to 2020, and the impact of the COVID-19 pandemic on influenza circulation. [Internet]. Geneva: World Health Organisation; 2021. [Published 2021-06-25, cited 2021-09-23]. Available from: https://www.who.int/publications/i/item/who-wer-9625-241-264
- Public Health Agency of Sweden, Rekommendationer om influensavaccination till riskgrupper, [Recommendations for influenza vaccination of risk groups] Stockholm: Public Health Agency of Sweden; September, 2020. [cited 2021-09-27] Available from: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/r/rekommendationer-om-influensavaccination-till-riskgrupper/
- Public Health Agency of Sweden, Nya vacciner mot säsongsinfluensa [New Seasonal Influenza Vaccines]. Stockholm: Public Health Agency of Sweden; March 12, 2021. [cited 2021-08-25] Available from: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/n/nya-vacciner-mot-sasongsinfluensa/
- Public Health Agency of Sweden, Erfarenheter samt förstärkt kommunikation vid vaccination mot säsongsinfluensa 2020/21 – Lärdomar att ta med till säsongsinfluensavaccination och vaccination mot covid-19 [Experiences of and reinforced communication about influenza vaccination 2020/21 – Lessons learned for seasonal influenza vaccination and vaccination against COVID-19]. Stockholm: Public Health Agency of Sweden; 2021 [Published 2021-06-03, cited 2021-09-27] Available from: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/e/erfarenheter-samt-forstarkt-kommunikation-vid-vaccination-mot-sasongsinfluensa-2020-2021/
- Public Health Agency of Sweden, Aktuell veckorapport om covid-19 [Current Weekly COVID-19 Report]. [Internet]. Stockholm: Public Health Agency of Sweden; 2021 [cited 2021-08-25] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/covid-19-veckorapporter/senaste-covidrapporten/
- World Health Organization, Recommended composition of influenza virus vaccines for use in the 2021-2022 northern hemisphere influenza season. [Internet]. Geneva: World Health Organization; 2021. [published 2021-02-26, cited 2021-09-02]. Available from: https://www.who.int/publications/i/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2021-2022-northern-hemisphere-influenza-season
- World Health Organization, Recommended composition of influenza virus vaccines for use in the 2020-2021 northern hemisphere influenza season. [Internet]. Geneva: World Health Organization; 2020. [published 2020-02-28, cited 2021-09-02]. Available from: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2020-2021-northern-hemisphere-influenza-season
Appendices
Appendices 1 and 2 are available upon request. Please provide the article number and the title of the report. The registrar will notify you if your document request entails a fee.
- Appendix 1. Influenza B/Victoria
- Appendix 2. Influenza A/H3N2
