
Influenza in Sweden – Season 2019–2020
Summary
The 2019–2020 influenza season was mild, and three viruses circulated: influenza A(H3N2), influenza A(H1N1)pdm09, and B/Victoria. The epidemic started in early December (week 49) and continued at low intensity for 16 weeks, with a peak in week 10. Following the implementation of measures to reduce the spread of COVID-19 in weeks 11-12, the number of cases dropped quickly and the epidemic came to an end in week 13 despite an increase in testing for influenza during this period. In the past 20 years, no season has gone from peak activity to end-of-epidemic as fast.
During the 2019–2020 season, 7,941 laboratory-confirmed cases were reported, of which the majority were influenza A (69 percent). Of the subtyped influenza A-samples, 64 percent were A(H3N2) and 36 percent were A(H1N1)pdm09. Samples of influenza B assigned to a lineage were exclusively B/Victoria.
The majority of cases overall were among individuals aged 0–39 years. However, the majority of influenza A cases were in individuals aged 40 years and older. The youngest children aged 0 to 4 years had the highest cumulative (146 per 100,000 population) and weekly incidence of influenza, followed by individuals aged 65 and older (105 per 100,000 population). The median age for individuals with laboratory-confirmed influenza A and B were 51 and 24 years, respectively.
Web searches reflected a low level of influenza activity during the season. Phone calls to the medical advice line 1177 indicated a medium level of activity during weeks 6 to 10. Both systems showed a sharp drop in activity towards the end of the season, similarly to the laboratory data.
During the season, 175 patients with influenza were reported as having received intensive care across the country, which is fewer than during the previous four seasons. The majority of patients (80 percent) had influenza A, with a median age of 61 years. The median age for patients with influenza B was 15, which is unusually low, and 20 out of 34 patients with influenza B were under 18 years of age. Samples were subtyped for 23 percent of patients with influenza A, and the results showed that 75 percent had influenza A(H1N1)pdm09. Of all reported cases in intensive care, 62 percent were in a risk group for severe influenza illness, either due to age (65 years and older) or due to one or more medical risk factors. The age distribution of patients in intensive care was most similar to the 2016–2017 season, which was dominated by influenza A(H1N1)pdm09.
During the 2019–2020 influenza epidemic, influenza-related excess mortality was measured during weeks 7 to 12 (FluMoMo model) in the age group 65 and older, followed by excess mortality due to COVID-19 from week 13 (see weekly reports for COVID-19). Among patients who received a laboratory-confirmed influenza diagnosis, 3 percent died within 30 days, which is similar to previous mild seasons; the percentage who died has ranged from 3 to 5.5 percent during the previous four seasons. In total, 89 percent of deaths were among people aged 65 years and older.
Weekly reports for COVID-19 (folkhalsomyndigheten.se)
In sentinel surveillance, influenza was detected in 18 percent of all samples. Of these, 57 percent were influenza A and 43 percent were influenza B. As in the laboratory-based reporting, both influenza A(H1N1)pdm09 and influenza A(H3N2) circulated from the start of the epidemic (week 48) until week 5. From week 6 onwards, influenza A(H3N2) dominated. Influenza B/Victoria circulated during the whole season. Cumulatively, the distribution was 33 percent A(H1N1)pdm09, 23 percent A(H3N2), and 43 percent B/Victoria. Vaccine break-through infections were detected in 7 percent of patients with influenza A(H1N1)pdm09 (median age 58 years) and 7 percent of patients with influenza A(H3N2) (median age 82 years).
The Public Health Agency participates in the European Influenza Monitoring Vaccine Effectiveness (I-MOVE) network with data from Swedish sentinel sampling. In the interim report for the 2019–2020 season, the vaccination effect was 48 to 75 percent for influenza A(H1N1)pdm09, <>0 to 57 percent for influenza A(H3N2), and 62 to 83 percent for influenza B (1).
During the 2019–2020 season, the average vaccination coverage among people 65 years and older was 53 percent, compared to 52 percent in the previous season. Due to delays in vaccine production, the 2019–2020 vaccination campaign started two weeks later than usual (19 Nov, week 47). The shortened period of vaccination does not seem to have affected the coverage rate among people 65 years and older.
Genetic characterisation of a subset of viruses collected through sentinel sampling and from laboratories around Sweden showed that of the characterised influenza A(H3N2) viruses, the majority (72 percent) belonged to one of three groups within the 3C.2a1b subgroup, while the remaining 28 percent belonged to subgroup 3C.3a. Of the characterised influenza A(H1N1)pdm09 viruses, the majority (92 percent) belonged to the 6B.1A5A subgroup, with 30 percent of these viruses having the amino acid substitution N156K, and 45 percent having the D187A+Q189E substitutions. The remaining A(H1N1)pdm09 viruses belonged to subgroup 6B.1A5B or 6B.1A7. Of the characterised influenza B/Victoria viruses, the vast majority (99 percent) belonged to subgroup 1Adel162-164B.
Three influenza A(H1N1)pdm09 viruses with the H275Y mutation known to confer clinical resistance to oseltamivir (Tamiflu/Ebilfumin) were detected. In one of these three viruses, a minority population with the N295S substitution, associated with reduced or highly reduced inhibition to oseltamivir, was present, along with the H275Y substitution, which was present in the vast majority of the virus population. The remaining 289 influenza A and B viruses for which the NA gene was sequenced did not carry any amino acid substitution known to confer reduced or highly reduced inhibition to the neuraminidase inhibitors oseltamivir (Tamiflu/Ebilfumin) or zanamivir (Relenza). An additional 94 A(H1N1)pdm09 viruses were analysed exclusively for the H275Y substitution, and none of these viruses carried this substitution.
Sammanfattning
Influensasäsongen 2019–2020 var mild och tre influensavirus cirkulerade: influensa A(H3N2), influensa A(H1N1)pdm09 och influensa B/Victoria. Epidemin började i början av december (vecka 49) och fortsatte med låg intensitet i 16 veckor, med en topp vecka 10. Efter åtgärderna för att minska spridningen av covid-19 sattes in under veckorna 11–12 minskade antalet influensafall snabbt och epidemin avslutades vecka 13, trots att provtagningen ökade under denna period. Under de senaste 20 åren har ingen säsong gått från toppaktivitet till avslut så snabbt.
Under säsongen 2019–2020 rapporterades 7 941 laboratoriebekräftade fall, varav majoriteten var influensa A (69 procent). Av de subtypade influensa A-proverna var 64 procent influensa A(H3N2) och 36 procent var influensa A(H1N1)pdm09. De influensa-B prover som linjetypats var uteslutande B/Victoria.
Majoriteten av fallen var bland personer i åldrarna 0–39 år men majoriteten av influensa A-fallen var bland personer 40 år och äldre. Barnen 0 till 4 år hade den högsta kumulativa (146 per 100 000 invånare) och veckovisa incidensen, följt av personer 65 år och äldre (105 per 100 000 invånare). Medianåldern för personer med laboratoriebekräftad influensa A och influensa B var 51 år respektive 24 år.
Webbsök för influensa visade på en låg influensaaktivitet under säsongen. Data över samtal till 1177 Vårdguiden nådde en medelhög aktivitet veckorna 6 till 10. Båda systemen visade en kraftig nedgång av aktiviteten mot slutet av säsongen, i likhet med laboratoriefallen.
Under säsongen rapporterades 175 patienter som intensivvårdades med laboratoriebekräftad influensa, vilket är färre än under de fyra föregående säsongerna. Majoriteten av patienterna (80 procent) hade influensa A, med en medianålder på 61 år. Prover subtypades för 23 procent av patienterna som intensivvårdats med influensa A och resultaten visade att 75 procent hade influensa A(H1N1)pdm09. Medianåldern för patienter med influensa B var 15, vilket är ovanligt lågt, och 20 av 34 patienter med influensa B var under 18 år. Av alla rapporterade intensivvårdade patienter tillhörde 62 procent en riskgrupp för svår influensasjukdom, antingen på grund av ålder (65 år eller äldre) eller på grund av en eller flera medicinska riskfaktorer. Åldersfördelningen för intensivvårdade patienter liknade säsongen 2016–2017, som dominerades av influensa A(H1N1)pdm09.
Under influensasäsongen 2019–2020 sågs en influensarelaterad överdödlighet under veckorna 7 till 12 (FluMoMo-modellen) i åldersgruppen 65 år och äldre, åtföljt av överdödlighet på grund av covid-19 från vecka 13 (se veckorapporterna för covid-19). Bland personer som fått en laboratoriebekräftad influensadiagnos hade 3 procent avlidit inom 30 dagar, vilket är i nivå med föregående säsonger med mild influensaktivitet. Andelen avlidna har varierat mellan 3 till 5,5 procent under föregående fyra säsonger. Totalt var 89 procent av dödsfallen bland personer 65 år och äldre.
Inom sentinelprovtagning påvisades influensa i totalt 18 procent av proverna vilket är lägre än föregående säsong. Influensa A stod för totalt 57 procent av de positiva proverna. Från epidemistart vecka 48 till och med vecka 5 cirkulerade både influensa A(H1N1)pdm09 och A(H3N2). Från och med vecka 6 dominerade influensa A(H3N2). Influensa B/Victoria påvisades under hela säsongen och andelen B var som högst veckorna 4–8. Sett över hela säsongen var 33 procent A(H1N1)pdm09, 23 procent A(H3N2) och 43 procent B/Victoria. Vaccinations-genombrott påvisades hos 7 procent av patienterna med A(H1N1)pdm09 (medianålder 58 år) och 7 procent av de med A(H3N2) (medianålder 82 år).
Folkhälsomyndigheten deltar i det europeiska nätverket för att mäta influensavaccinets effekt I-MOVE med data från den svenska sentinelprovtagningen. Vid interimsrapporten för säsong 2019–2020 var vaccinationseffekten 48 till 75 procent för influensa A(H1N1)pdm09, <0 till 57 procent för influensa a(h3n2) och 62 till 83 procent för influensa b (1).>
Under säsongen 2019–2020 var vaccinationstäckningen bland personer 65 år och äldre i medel 53 procent nationellt, jämfört med 52 procent föregående säsong. På grund av förseningar i vaccinproduktionen startade vaccinationskampanjen två veckor senare än vanligt, den 19 november (vecka 47). Den förkortade perioden för vaccinationer verkar inte ha påverkat vaccinationstäckningen bland personer 65 år och äldre.
Viruskaraktäriseringen har gjorts på ett urval av de stammar som samlats in genom sentinelprovtagningen och från laboratorier i landet. Majoriteten (72 procent) av de karaktäriserade A(H3N2) -stammarna tillhörde samtliga någon av tre undergrupper till genetisk subgrupp 3C.2a1b. Resterande 28 procent av stammarna tillhörde subgrupp 3C.3a. Av de analyserade A(H1N1)pdm09-stammarna tillhörde majoriteten (92 procent) genetisk subgrupp 6B.1A5A, där 30 procent hade aminosyrautbytet N156K, och 45 procent utbytena D187A+Q189E. Resterande A(H1N1)pdm09-stammar tillhörde subgrupp 6B.1A5B eller 6B.1A7. Majoriteten (99 procent) av de analyserade B/Victoria-stammarna tillhörde genetisk subgrupp 1Adel162-164B.
Tre influensa A(H1N1)pdm09-stammar med aminosyramutationen H275Y som ger upphov till resistens mot oseltamivir (Tamiflu/Ebilfumin) påvisades. Hos en av stammarna påvisades förutom mutation H275Y (som fanns i den stora majoriteten av viruspopulationen hos den aktuella stammen), även en liten andel viruspopulation med mutation N295S, vilket är associerat med reducerad eller mycket reducerad känslighet för oseltamivir). Hos resterande 289 stammar påvisades inga av de aminosyramutationer som är kända för att ge upphov till reducerad eller mycket reducerad känslighet för oseltamivir eller zanamivir (Relenza). Ytterligare 94 stammar analyserades med realtids-PCR för enbart aminosyramutation H275Y. Ingen av dessa stammar bar på den aktuella mutationen.
About this publication
This report describes the monitoring systems for influenza in use during the winter season of 2019–2020 and the results of both epidemiological and virological surveillance. Data are also compared to previous influenza seasons.
The report has been prepared for the World Health Organization (WHO) as part of the Public Health Agency of Sweden’s function as a National Influenza Centre (NIC).
Annual reports in English about the influenza seasons in Sweden have been available since 1997, and those from 2000–2001 onward can be found on the Public Health Agency’s website (suggested search “Influenza in Sweden”) (2).
Public Health Agency of Sweden
Mia Brytting
Head of Unit
Unit for Laboratory Surveillance of Viral
Pathogens and Vaccine Preventable Diseases
Sören Andersson
Head of Unit
Unit for Vaccination Programs
Influenza surveillance in Sweden
Influenza epidemics recur in Sweden each winter, with a range of effects depending on the characteristics of the circulating viruses and the level of immunity in different age groups. New influenza strains, particularly those different enough to cause a pandemic, can cause a high number of severe cases, which can cause great strain on intensive care units and can lead to deaths in all age groups. None of these effects are detectable through a single reporting system. In order to get an overall picture of on-going influenza activity and to remain prepared in case of an influenza pandemic, the Public Health Agency of Sweden (Folkhälsomyndigheten) has a number of different epidemiological reporting systems for influenza ranging from the collection of data from different healthcare providers to the analysis of web searches.
Virological surveillance is as important as epidemiological reporting systems. Viruses are typed as influenza A or B by regional laboratories in real time during the influenza season, and some laboratories also determine the subtype for influenza A. Throughout the season, viruses from around the country are characterised by the Public Health Agency with regard to subtype and lineage, vaccine similarity, sensitivity to antiviral drugs, and other factors that might affect the severity of the infections they cause. Viruses are also isolated and sent to the WHO Collaborating Centre (WHO CC) in London for further characterisation and to provide a basis for vaccine strain selection. When new strains of influenza virus emerge, reference methods for diagnostics are established at the Public Health Agency and shared with all microbiological laboratories in Sweden.
During the influenza season, the Public Health Agency condenses national and international data into a detailed weekly bulletin that is published on the agency’s website (3). A preliminary summary of the 2019–2020 season was published on June 25, 2020 (4). The bulletin provides timely analysis of the current situation in Sweden and abroad and has a wide readership.
Updates to national policies and recommendations
During the 2019–2020 season, updated web-based information about vaccination, influenza, and pandemic preparedness were published continuously throughout the season. In addition, the new pandemic preparedness plans, which can be found at the Public Health Agency website under Pandemic Preparedness (9), were published:
- Pandemic Preparedness: How We Prepare
- Pandemic Preparedness: How We Communicate
- Pandemic Preparedness: Guidance on Access and Use of Medical Countermeasures
Surveillance 2019–2020
The pyramid below illustrates the different outcomes and levels of severity and the corresponding surveillance systems for individuals infected with influenza (Figure 1), ranging from the large number of infected individuals to the small number who die as a result of the influenza infection. The Public Health Agency used a number of data sources to monitor influenza activity at the various levels of the influenza pyramid in Sweden during the 2019–2020 season. Each system is described at the start of each section in the report with the season’s results in detail. The COVID-19 pandemic had a significant impact on influenza surveillance from March 2020 to the end of the surveillance period (week 20), with changes to healthcare seeking and testing behaviour, increased calls to medical advice lines, and reprioritisation within the nation’s laboratories.
Table 1 shows at which week each system, as applicable, crossed the threshold for epidemic start, reached its peak notation, and crossed the threshold for the end of the epidemic, as well as the maximum intensity level measured by the system during the season.
Figure 1. The “influenza pyramid” showing possible outcomes of an influenza infection and the surveillance systems at each level.
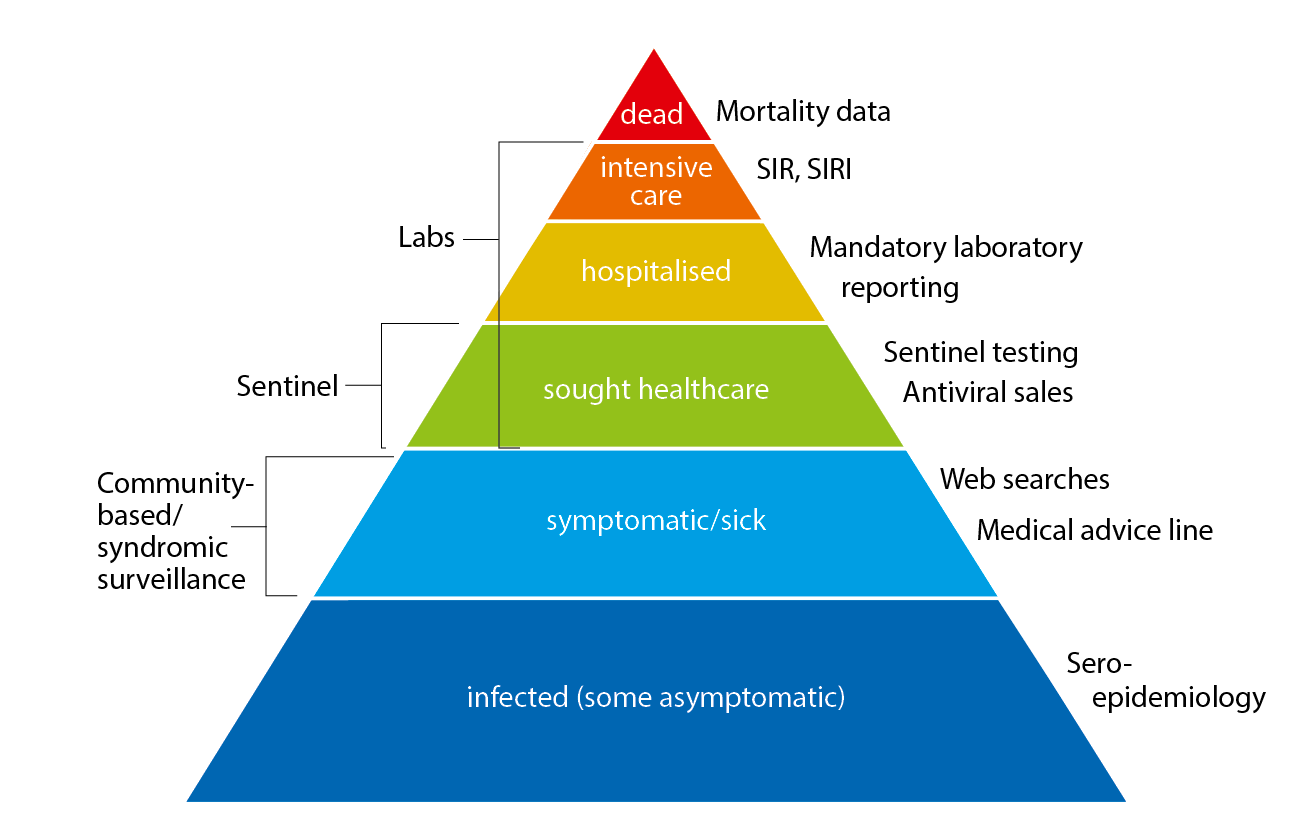
SIR: Swedish Intensive Care Registry. SIRI: Swedish Intensive Care Registry – Influenza module.
Summary of genetic characterisation 2019–2020
Of 79 analysed A(H3N2) viruses, 72 percent belonged to one of three groups of the 3C.2a1b subgroup. Subgroup 3C.1ab viruses have shown poor antigenic similarity to the vaccine virus for the northern hemisphere season 2019-2020. The remaining 28 percent of the A(H3N2) viruses belonged to subgroup 3C.3a, i.e. the same subgroup as the vaccine virus, and viruses in this subgroup have shown good antigenic similarity to cell-propagated vaccine virus, but less similarity to egg-propagated vaccine virus.
Of 94 analysed A(H1N1)pdm09 viruses, 92 percent belonged to genetic subgroup 6B.1A5A, a group in which the majority of the viruses have shown good antigenic similarity to the vaccine virus in genetic subgroup 6B.1A1. However, the amino acid substitution in position N156K present in 30 percent of the characterised Swedish 6B.1A5A viruses have been shown to result in poor antigenic similarity to the vaccine virus. An additional 45 percent of the 6B.1A5A viruses carried the D187A+Q189E substitutions. Human serological analyses have shown results indicative of antigenic drift of 6B.1A viruses, including those in subgroup 6B.1A5A with D187A+Q189E.
All but one of the 114 analysed B/Victoria viruses belonged to genetic subgroup 1Adel162-164B, a subgroup in which the vast majority of viruses have shown poor antigenic similarity to both cell and egg-propagated vaccine virus (in subgroup 1Adel162-163).
Genotypic antiviral susceptibility testing
The neuraminidase gene of 292 influenza A and B viruses has been sequenced. Three A(H1N1)pdm09 viruses were shown to carry the H275Y substitution (associated with oseltamivir resistance). One of these three viruses also had the N295S substitution (in the minority), associated with reduced or highly reduced inhibition to oseltamivir, along with the H275Y substitution (which was present in the vast majority of the virus population). An additional 94 A(H1N1)pdm09 viruses were analysed exclusively for the H275Y substitution. None of these viruses carried this substitution.
Phenotypic antiviral susceptibility testing
A total of 28 viruses were analysed by The Public Health Agency Sweden and/or WHO CC in London, and all viruses except the A(H1N1)pdm09 virus with the amino acid substitutions H275Y (in the vast majority)+N295S (in the minority) were shown to be sensitive to both oseltamivir and zanamivir. The virus with the H275Y+N295S substitutions showed highly reduced inhibition to oseltamivir and normal inhibition to zanamivir.
| System | Start | Peak | End | Max intensity |
|---|---|---|---|---|
| Laboratory-based surveillance (number of cases) | 49 | 10 | 13 | Low |
| Laboratory-based surveillance (percent positive) | 49 | 9 | 12 | Low |
| Antiviral sales | (a) | 9, 11-12 | (a) | (a) |
| Laboratory-confirmed influenza cases in intensive care (SIRI) | (a) | 1, 5 | (a) | (a) |
| Excess mortality | 7 | 11 | 12 | (a) |
| Sentinel sampling | 48 | 6 | 12 | (a) |
| Webbsök (Web Search) | 44 | 52, 9-11 | 13 | Low |
| Telephone Advice Line (1177 Vårdguiden) | 50 | 7-8 | 13 | Medium |
(a) Epidemic thresholds/intensity levels not assigned.
Laboratory-based surveillance
All laboratory-confirmed cases of influenza have statutory reporting requirements (as of Dec 1, 2015), but subtyping is not required. Denominator data (the total number of samples analysed) are reported voluntarily via e-mail. Samples analysed for influenza at the laboratories in Sweden have historically primarily been done in hospital settings, but the proportion of cases reported from outpatient settings has increased in recent years.
The 2019–2020 influenza season was mild, and three viruses circulated: influenza A(H3N2), influenza A(H1N1)pdm09, and B/Victoria. The epidemic started in week 49 (early December) and continued for 16 weeks until it ended in week 13. As in previous seasons, the number of laboratory-confirmed influenza cases increased throughout December and then decreased during January. A steady increase in the number of cases was seen from week 4 leading to the peak in week 10 when 888 cases were reported (Figure 2). Following the implementation of measures to reduce the spread of COVID-19 in weeks 11-12, the number of cases dropped quickly and the epidemic came to an end in week 13. The percentage positive decreased from 17 percent in week 11 to less than 1 percent in week 14 (Figure 3). The rapid decrease of the influenza epidemic occurred despite an increase in testing for influenza during this period. In the past 20 years, no season has gone from peak activity to end-of-epidemic as fast.
The majority of the cases (65 percent) during the peak (week 10) were influenza A and based on the samples that were subtyped influenza A(H3N2) was the dominating subtype in the laboratory-based surveillance system during the season overall. There was an outbreak of influenza A(H1N1)pdm09 in the northern part of the country at the beginning of the season, but thereafter only a small number of influenza A(H1N1)pdm09 cases were reported in comparison to influenza A(H3N2). In Sweden, influenza A most recently dominated the seasons during 2018–2019 and 2016–2017, when influenza A(H1N1)pdm09 and A(H3N2) circulated, respectively. See also Subtyping and lineage determination.
In comparison with recent seasons, the number of reported laboratory-confirmed influenza cases during the current season (7,941) was lower than the last three seasons, see Table 2. Although similar to the number of cases reported in 2015–2016, this season was milder, as seen in the lower percentage positive samples this season (10 percent compared to 19 percent during 2015–2016). During the peak week (week 10), the number of cases was lower than the peak in recent seasons (Figure 2). Fewer samples were taken during the 2019–2020 season in comparison to the two previous seasons, and overall 10.5 percent of the samples taken were positive for influenza, which is lower compared to previous seasons, in part reflecting the lower intensity of the season (Table 2, Figure 3).
The proportions of samples taken at outpatient settings have increased during the last three seasons, from 60 percent in 2017–2018 to 72 percent in 2019–2020, as virological analyses have become faster and more widely available (Figure 4).
Figure 2. Total number of laboratory-confirmed cases of influenza (all types) per week and the dominating influenza type(s) per season, 2015–2020.
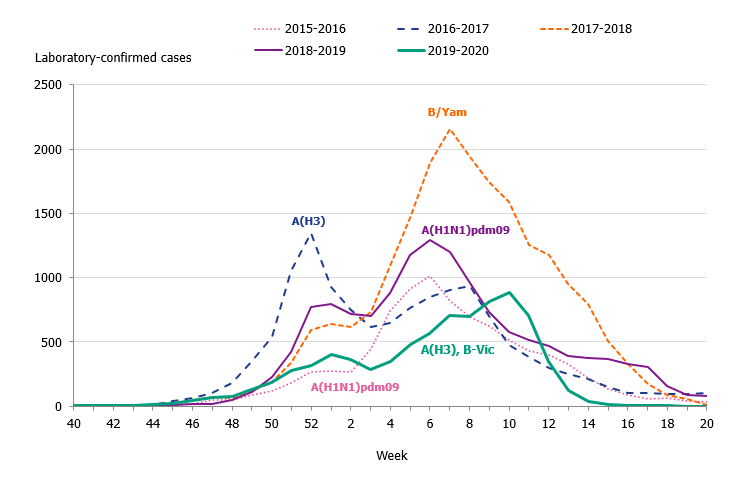
Figure 3. Percentage of samples testing positive for influenza, per week, 2015–2020.
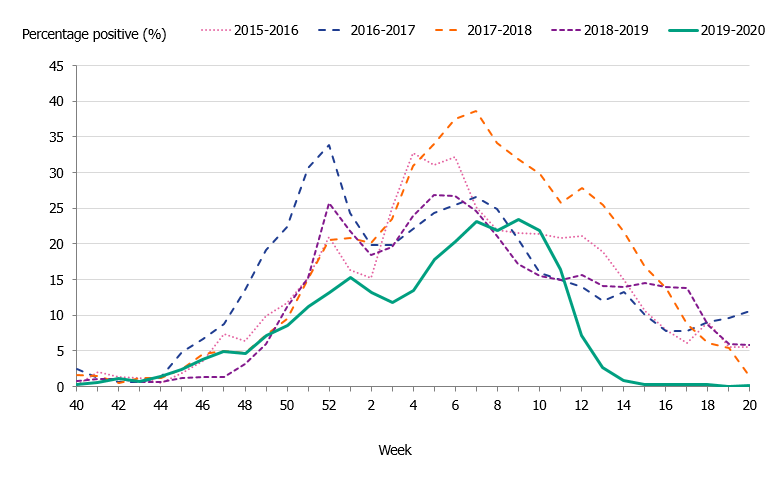
Figure 4. Number of laboratory-confirmed influenza cases stratified by level of care at sampling, 2015–2016 to 2019–2020.
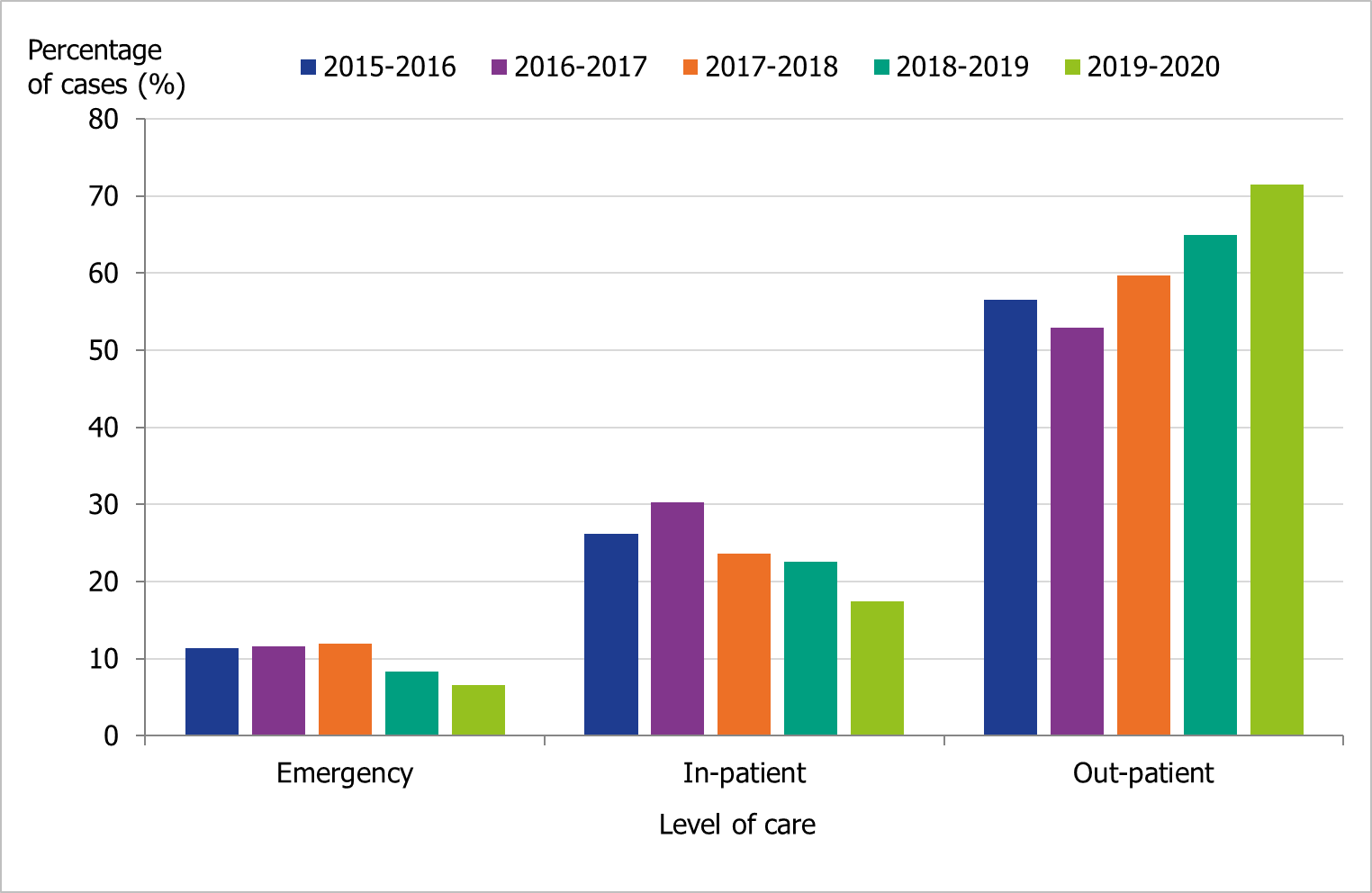 Level of care could not be determined for a small number of cases, and these have been excluded.
Level of care could not be determined for a small number of cases, and these have been excluded.
Viral distribution
During the 2019–2020 season, 7,941 laboratory confirmed cases were reported, of which the majority was influenza A (69 percent). Table 2 summarises the laboratory reporting results over the last five seasons, including the number of analysed samples and the proportion of positive samples as well as the total samples positive by type.
| Indicator | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|---|---|
| Analysed samples | 48,135 | 68,241 | 88,837 | 83,325 | 75,819 |
| Proportion positive samples (percent) | 19 | 19 | 23 | 17 | 10 |
| Total positive for influenza A | 6,730 | 12,361 | 7,406 | 13,664 | 5,441 |
| Total positive for influenza B | 2,409 | 708 | 13,280 | 93 | 2,500 |
Age and sex distribution
During the 2019–2020 season, the majority (52 percent) of the laboratory-confirmed influenza A and B cases were among individuals aged 0-39 years. However, individuals aged 40 and older had the majority (61 percent) of the influenza A cases (Table 3).
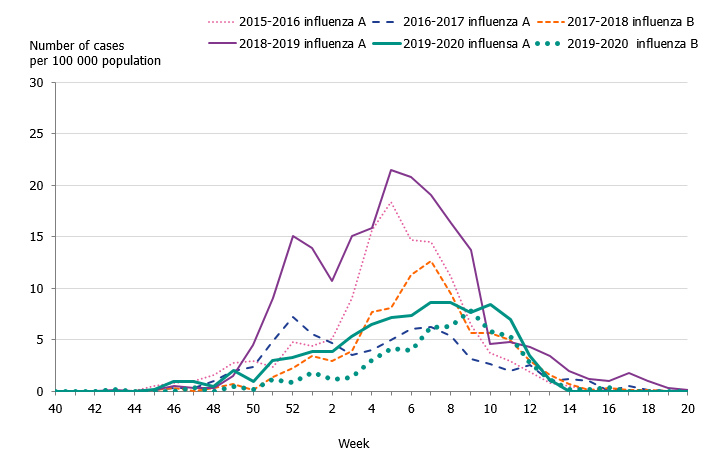
Children aged 0 to 4 years had the highest cumulative (146 per 100,000 population) and weekly incidence of influenza, followed by individuals aged 65 and older (105 per 100,000 population, Figure 5, Table 4). The incidence for individuals aged 65 and older was lower compared to the two previous seasons when influenza A(H3N2) and influenza B/Yamagata circulated, respectively (Figure 6). Although the weekly incidence was highest among the youngest children during the current season, the incidence was lower compared to the two previous seasons, which were more intense (Figure 7). The youngest cohorts of children who have not previously been infected by the circulating virus are at risk for infection each season. However, children who become infected by influenza usually recover at home and do not need to seek medical care for influenza. The age distribution of the laboratory-confirmed cases thus only reflects the burden in terms of children who have sought care for their influenza infection.
The age distribution of the current season is most similar to 2015–2016, during which influenza A(H1N1)pdm09 dominated but influenza B/Victoria also circulated to a minor extent (see Table 3), which reflects the fact that the season saw the circulation of three influenza viruses. This is also seen in the median age of individuals with laboratory-confirmed influenza A (51 years, see Table 5). Significantly more women (52 percent) than men (48 percent) had laboratory-confirmed influenza (p < 0.001).
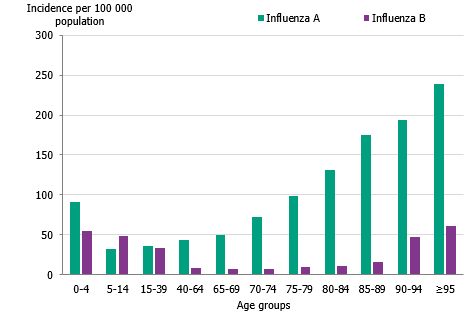
The majority of the influenza B laboratory-confirmed cases were seen among individuals aged 15 to 39 years (43 percent), followed by individuals aged 5 to 14 years (14 percent). The median age for influenza B was 24 years, which was expected because B/Victoria usually affects children and young adults more than other age groups and has not circulated to a great extent in recent seasons. The incidence of laboratory-confirmed influenza B was highest in the youngest age groups as well as those 90 years and above (Figure 8, Table 4). The incidence for influenza A per age group showed a u-shaped curve, with a higher incidence among children 0–4 years, which subsequently decreased for age groups up until 65-69 years of age, and thereafter increased steadily, reflecting those age groups usually in need of medical care and therefore receiving a laboratory diagnosis.
Figure 5. Weekly incidence of influenza A and B per age group in Sweden, 2019–2020 season.
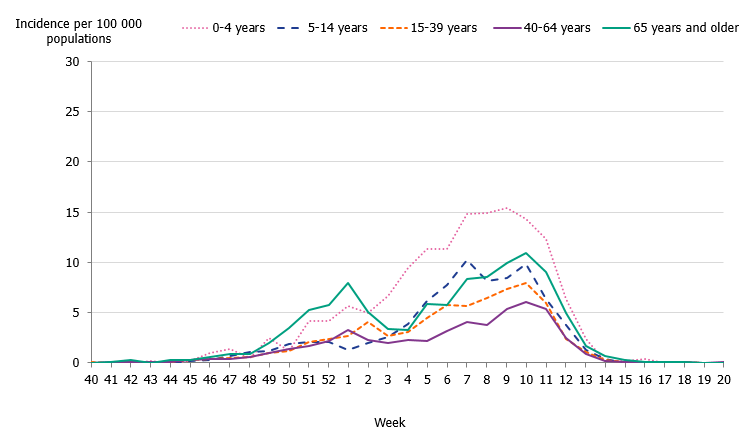
Figure 6. Weekly incidence of influenza (dominating type) for individuals aged 65 and older in Sweden, 2015–2020 seasons.
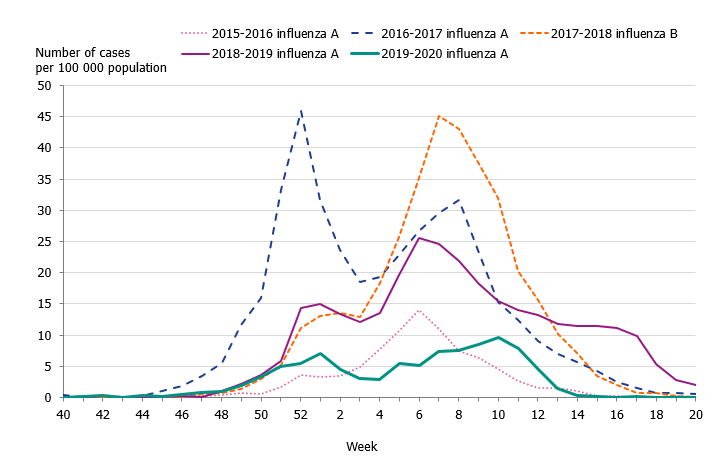
Figure 7. Weekly incidence of influenza (dominating type) for children aged 0–4 years in Sweden, 2015–2020 seasons.
| Age group | 2015-2016 Incidence | 2015-2016 Percentage | 2016-2017 Incidence | 2016-2017 Percentage | 2019–2020 Incidence | 2019–2020 Percentage |
|---|---|---|---|---|---|---|
| 0–4 | 130 | 11 | 73 | 4 | 91 | 10 |
| 5–14 | 26 | 4 | 30 | 3 | 33 | 7 |
| 15–39 | 53 | 25 | 45 | 11 | 36 | 21 |
| 40–64 | 71 | 33 | 69 | 17 | 43 | 25 |
| 65+ | 93 | 27 | 405 | 65 | 94 | 36 |
| Total | 682 | 100 | 124 | 100 | 53 | 100 |
Note: Data do not include sentinel cases or cases where the age is unknown.
Figure 8. Incidence (per 100,000 population) of laboratory-confirmed influenza cases per age group and influenza type, Sweden, 2019–2020.
The table (Table 4) does not include sentinel cases or cases where age is unknown. Population on December 31, 2019. Source: Statistics Sweden, Statistikdatabasen.
| Age group | Population (b) | Influenza A Cases | Influenza A Incidence | Influenza B Cases | Influenza B Incidence | Incidence (all influenza) |
|---|---|---|---|---|---|---|
| 0–4 | 601,718 | 547 | 91 | 329 | 55 | 146 |
| 5–14 | 1,233,103 | 402 | 33 | 602 | 49 | 81 |
| 15–39 | 3,262,793 | 1,161 | 36 | 1,078 | 33 | 69 |
| 40–64 | 3,164,608 | 1,373 | 43 | 257 | 8 | 52 |
| 65–69 | 540,048 | 269 | 50 | 38 | 7 | 57 |
| 70–74 | 560,415 | 405 | 72 | 42 | 7 | 80 |
| 75–79 | 428,598 | 425 | 99 | 42 | 10 | 109 |
| 80–84 | 273,050 | 357 | 131 | 31 | 11 | 142 |
| 85–89 | 163,629 | 286 | 175 | 26 | 16 | 191 |
| 90–94 | 76,644 | 149 | 194 | 36 | 47 | 241 |
| ≥95 | 22,983 | 55 | 239 | 14 | 61 | 300 |
| Total | 10,327,589 | 5,429 | 53 | 2,495 | 24 | 77 |
| Indicator | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|---|---|
| Dominant type | A(H1N1)pdm09 | A(H3N2) | B/Yamagata | A(H1N1)pdm09 | Mixed season |
| Median age influenza A | 47 | 73 | 70 | 60 | 51 |
| Median age influenza B | 33 | 58 | 68 | 37 | 24 |
Geographic distribution
During the initial weeks of the epidemic, the northern parts of Sweden (Norrland) had higher incidence in comparison to the middle (Svealand) and southern (Götaland) parts of the country (Figure 9). During the Christmas and New Year holidays the influenza activity levelled out and was similar across the country. In February, the incidence increased. The incidence reached a peak in the northern parts of the country (Norrland) in week 9 followed by peaks for the southern (Götaland) and the middle (Svealand) parts of the country in week 10. The percentage of samples positive for influenza had peaks in week 10 for the northern part of the country (Norrland), whereas the middle (Svealand) parts of the country peaked during weeks 6-9 and southern (Götaland) parts peaked in week 7.
Altogether, the northern parts of the country (Norrland) had the highest cumulative incidence of laboratory-confirmed cases per 100,000 individuals this season, with 113 cases, followed by the middle parts (Svealand) with 77 cases and the southern parts (Götaland) with 68 cases. The greatest numbers of cases were reported from the largest urban areas (Stockholm, Västra Götaland, and Skåne). However, in relation to population size, three counties in the northern part of the country had the highest cumulative incidence (Västernorrland, Norrbotten, Västerbotten). The number of laboratory-confirmed cases might be affected by healthcare-seeking behaviour as well as differences in sampling in the various regions; thus, no direct conclusions can be drawn regarding actual influenza activity using the measured incidence.
Figure 9. Weekly incidence of laboratory-confirmed influenza per 100,000 population and county from week 40, 2019, to week 20, 2020.

Antiviral sales
Every Monday, the Public Health Agency receives data from the Swedish eHealth Agency on the previous week's sales of the antivirals zanamivir and oseltamivir. Data include all sales categories, i.e. prescriptions and healthcare requisitions. The number of prescriptions directly reflects patients seeking medical care for influenza-like symptoms, whereas requisitions more likely show medical staff preparations for intense periods as well as reflect patients currently in treatment.
Cumulative sales of antiviral medications were at lower levels compared to the last three seasons. During the 2019–2020 season, sales first increased steeply toward the end of December (week 51), ahead of the Christmas holidays, despite the fact that the influenza cases were still at low levels. This increase primarily reflected increased requisitions from hospitals and long-term care facilities. After this period, sales followed the pattern of influenza cases and increased again to another, broader peak around the influenza peak at week 10.
During the 2019–2020 season, sales of antivirals were much higher than the number of influenza cases (Table 6). Antiviral sales might be increasing (when compared to influenza cases) partly due to increased efforts by several county councils to make rapid and reliable influenza diagnostics available, meaning doctors have the opportunity to start antiviral treatment quickly and thus with better treatment effects. After the start of the season, requisitions increased sharply, likely due to hospitals and other facilities preparing for the peak of the season, which in turn was lower than expected and ended faster than expected due to actions taken against COVID-19.
| Indicator | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|---|---|
| Prescriptions | 3,720 | 4,782 | 7,120 | 6,086 | 5,263 |
| Health care requisitions | 4,930 | 10,144 | 12,790 | 10,534 | 7,252 |
| Total sales | 8,650 | 14,926 | 19,910 | 16,620 | 12,515 |
| Total influenza cases | 9,134 | 13,069 | 20,686 | 13,757 | 7,941 |
Influenza cases in intensive care
The Public Health Agency receives anonymised data daily on influenza patients in intensive care through a collaboration with the Swedish Intensive Care Registry (SIR). A special influenza module in the registry, known as SIRI, allows the treating physician at an intensive care unit to report underlying medical conditions, complications, antiviral treatment, vaccination status, influenza type and subtype, and other data for patients under treatment.
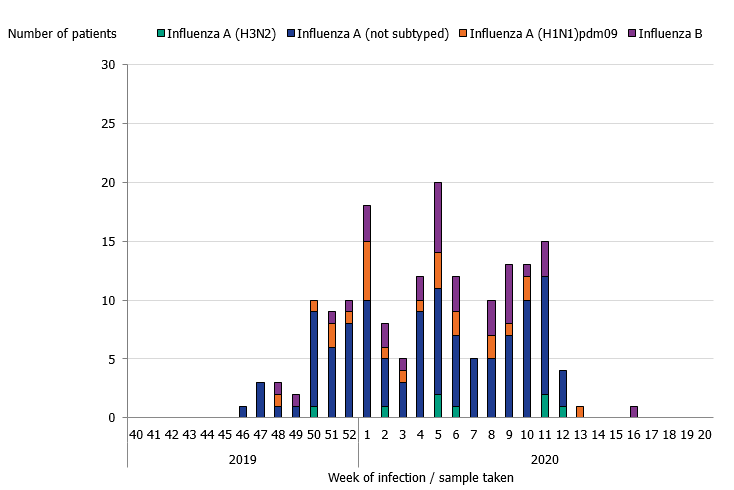
During the 2019–2020 season, 175 patients with influenza were reported as having received intensive care. The majority of the cases had influenza A (141 patients), and 34 patients had influenza B (Table 7 and Figure 10). Of patients with influenza A and reported subtype, 24 patients had influenza A(H1N1)pdm09 and 8 patients had influenza A(H3N2). The greatest number of patients were admitted to intensive care during week 5 (20 patients), which was several weeks before the peak laboratory-confirmed cases of influenza in week 10. In comparison to the previous seasons, the proportion of patients aged 39 and younger was higher. The age distribution of patients in intensive care was most similar to the 2015–2016 season, which was dominated by influenza A(H1N1)pdm09, despite influenza A(H3N2) being a higher proportion among laboratory-confirmed cases overall during the 2019–2020 season. Among the patients aged 39 years and younger, 27 patients had influenza A and 26 had influenza B. The median age was 61 years for patients with influenza A and 15 years for influenza B. The low median age for influenza B cases in intensive care is unusual. In total, 20 out of 34 patients were under 18 years of age. There was no significant difference in sex distribution.
Of all reported cases in intensive care, 109 patients (62 percent) were in a risk group for severe influenza illness, either due to age (65 years and older) or due to having one or more medical risk factors. Among patients under the age of 65 years, more than half (66 patients, 58 percent) did not have a medical risk factor for severe influenza. As in the previous three seasons, chronic heart-lung disease (n = 53) and immunosuppression (n = 26) were the most commonly reported risk factors in the 2019–2020 season along with diabetes (n = 18). None of the patients were pregnant.
Of the patients who were recommended vaccination, vaccination status was known for 84 patients, of which 17 (20 percent) were vaccinated. In addition, one patient without any reported risk factor was vaccinated. Of the vaccinated patients, 16 patients had influenza A and two influenza B. The age for vaccinated patients ranged from 1 to 89 years, with a median age of 71 years. The majority of the vaccinated patients were aged 65 years and older (14 patients).
Information regarding primary diagnosis in intensive care was reported for 171 patients. Influenza with pneumonia and influenza with other respiratory complications were the most common primary diagnoses for intensive care.
Of the patients requiring intensive care, 33 individuals were reported to have died. The majority (82 percent) of the deceased had a medical risk factor or were aged 65 years or older and therefore were at increased risk of severe influenza infection. The median age patients who died was 70 years.
Figure 10. Number of patients with influenza in intensive care by influenza type or subtype, 2018–2019 season.
| Samples | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|---|---|
| Influenza A (not subtyped) |
157 (56) | 196 (73) | 139 (64) | 316 (64) | 109 (58) |
| Influenza A(H1N1)pdm09 | 156 (58) | 3 (82) | 9 (59) | 35 (64) | 24 (63) |
| Influenza A(H3N2) | 4 (56) | 50 (72) | 13 (72) | 6 (66) | 8 (73) |
| Influenza B | 50 (52) | 9 (66) | 291 (67) | 2 (74) | 34 (15) |
| Total | 367 (57) | 258 (72) | 452 (66) | 359 (64) | 175 (55) |
Incidence of patients in intensive care with influenza
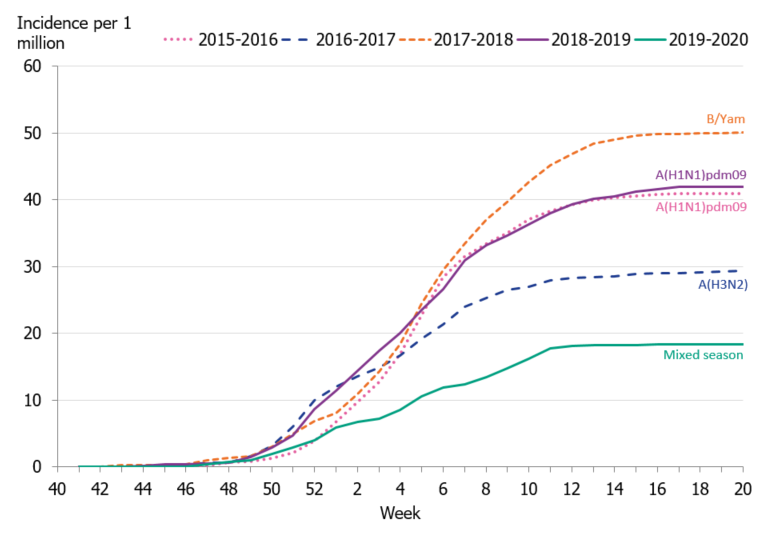
To enable comparison of the past seasons, we estimated the incidence of patients in intensive care with influenza. The incidence for the last four seasons was calculated based on preliminary estimates of regional catchment population denominators. The weekly incidence of the current season was lower in comparison to the previous four seasons, even during the peak weeks (Figure 11). The cumulative incidence was also lower than that of previous seasons; however, it is most similar to the 2016–2017 season when influenza A(H3N2) dominated (Table 8, Figure 12).
| Indicator | 2015–2016 | 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 |
|---|---|---|---|---|---|
| Total number of patients | 367 | 258 | 447 | 359 | 175 |
| Cumulative incidence per 1 million population | 50 | 29 | 50 | 42 | 18 |
| Reporting ICUs | 53 | 49 | 53 | 51 | 51 |
(a) Incidence calculations use preliminary estimates of regional catchment population denominators for each region based on information about which ICUs have reported each season.
Figure 11. Weekly incidence of patients with influenza in intensive care, last five seasons, 2015–2020.
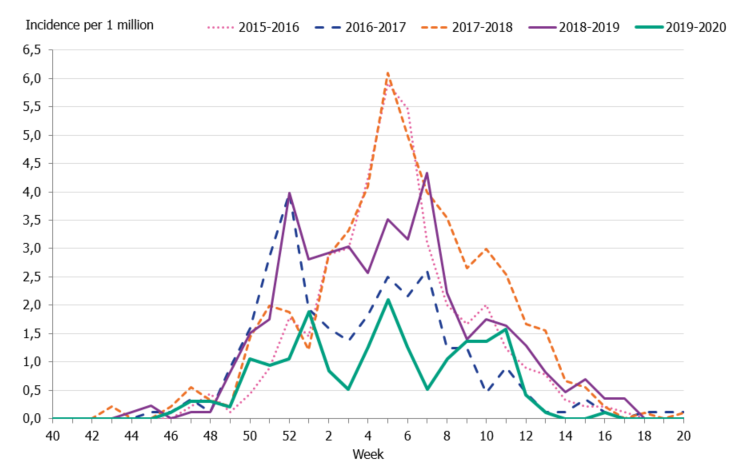
Figure 12. Cumulative incidence of patients with influenza in intensive care per week, last five seasons, 2015–2020.
Influenza-related mortality
Influenza-related mortality is often noted during influenza seasons and varies with circulating strains and the intensity of the season. The Public Health Agency uses different systems to measure influenza-related mortality.
Data on laboratory-confirmed influenza patients are intermittently linked to Swedish Tax Agency data on death in order to identify deceased individuals and to retrieve their dates of death. If 30 days or less have elapsed since the influenza diagnosis, the death is considered to be influenza related. This measurement is not specific nor is it sensitive. It is imprecise because a death within 30 days might have been caused by something else, and it is not sensitive because this measure does not include deaths if the diagnosis of influenza was not laboratory confirmed. Therefore, there are most likely a large number of unrecorded deaths from influenza. In addition, the data do not include information about underlying risk factors or complications of influenza infection. The analysis below includes data on cases reported until June 9, 2020 (week 19), not including 136 people without a national identification number.
To better estimate mortality, two models are used to identify crude and influenza-related excess mortality during the influenza season using the aggregate number of deaths. The EuroMoMo model estimates the crude excess mortality for the whole country by age group and regionally. The FluMoMo model estimates excess mortality due to either influenza activity or extreme temperatures, both nationally and by age group.
Deaths 30 days after influenza diagnosis
In total, 210 of 7,791 persons who received a laboratory-confirmed influenza diagnosis during the 2019–2020 season (to week 19) died within 30 days of diagnosis. This corresponds to 3 percent of the laboratory-confirmed cases, which is at the lower range compared to the previous four seasons for which this analysis has been possible (range 3–5.5 percent). Table 9 summarises the deaths within 30 days over the past four seasons. Within the 30 days, most (73 percent) of those who died did so within 15 days of diagnosis.
Of deaths occurring within 30 days of sampling, 89 percent of the cases had an influenza A diagnosis (186 people), while 11 percent had influenza B (24 people). As such, the percentage of deaths with influenza B was lower in comparison to the percentage among all laboratory-confirmed cases (regardless of outcome), where 31 percent were influenza B. During the season, all influenza B cases belonged to the B/Victoria lineage. The lower proportion of influenza B/Victoria among deaths reflects the fact that the oldest age group has some protection against this type of influenza. For most of those with influenza A who died within 30 days, no subtype was specified (154 people). Of the subtyped samples, 22 were influenza A(H3N2) and 10 were influenza A(H1N1)pdm09.
Patients who died had a median age of 81 years of age, and patients who did not die within 30 days of diagnosis had a median age of 36 years, which reflects the mixed circulation of viruses during the season affecting different age groups. During the season, influenza B/Victoria, A(H3N2), and A(H1N1)pdm09 co-circulated, which makes the season different from the other four available for comparison. In total, 8.5 percent of all people aged 65 and older who had a laboratory-verified influenza died within 30 days.
More men than women died within 30 days of an influenza A diagnosis (57 percent of the deaths were men), and the difference was statistically significant despite the fact that men accounted for a slightly lower proportion (48 percent) of the laboratory-confirmed cases during the season.
| Influenza season and dominant type | Cases | Deaths | Percent deceased | Percent of 65+ who died | Median age of deaths (years) | Median age of survivors (years) |
|---|---|---|---|---|---|---|
| 2015-2016, A(H1N1)pdm09 | 8 915 | 255 | 3 | 8 | 75 | 43 |
| 2016-2017, A(H3N2) | 13,087 | 734 | 6 | 8 | 85 | 72 |
| 2017-2018, B/Yamagata | 20,438 | 1,012 | 5 | 8 | 84 | 67 |
| 2018-2019, A(H1N1)pdm09 | 13,324 | 505 | 4 | 7 | 81 | 59 |
| 2019-2020, mixed season | 7,799 | 218 | 3 | 9 | 81 | 36 |
In total, approximately 87.5 percent of deaths within 30 days occurred among people aged 65 years and older, while 9.5 percent of deaths occurred among adults aged 40–64 years and 3 percent occurred in people under the age of 40 years, see Table 10. The table also shows that the proportion of deaths within 30 days increased with increasing age and varied from 0.1 percent for persons aged under 40 years to 22 percent for people 95 years and older. The analyses were not adjusted for expected mortality per age group.
| Indicator | <40 years> | 40–64 years | 65–69 years | 70–74 years | 75–79 years | 80–84 years | 85–89 years | 90–94 years | ≥95 years | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases | 4,038 | 1,597 | 301 | 443 | 462 | 385 | 311 | 185 | 69 | 7,791 |
| Cases/100,000 | 80 | 51 | 55 | 79 | 115 | 147 | 192 | 243 | 313 | 1,274 |
| Total Deaths | 6 | 20 | 10 | 21 | 27 | 43 | 33 | 35 | 15 | 210 |
| Deaths/100,000 | 0,1 | 1 | 2 | 4 | 7 | 16 | 20 | 46 | 68 | 164 |
| Deaths among cases | 0,1 % | 1 % | 3 % | 5 % | 6 % | 11 % | 11 % | 19 % | 22 % | 3 % |
This analysis includes all laboratory-confirmed influenza cases from week 40, 2019, to week 19, 2020. It excludes 140 patients whose personal identification number was not included in the case report, meaning that their status at 30 days could not be ascertained.
Excess mortality
During the 2019–2020 influenza season, influenza-related excess mortality was noted during the period when influenza was circulating. According to the FluMoMo model, influenza-related excess mortality reached just above the expected number of deaths in the age group 65 and older during weeks 7 to 12. This was followed by a period of excess mortality due to COVID-19, which can be seen in the figure below in grey (see weekly reports for COVID-19) from week 13. According to the EuroMoMo model, there was no excess mortality during the influenza epidemic.
Weekly reports for COVID-19 (in Swedish)
Influenza-related mortality is more often seen in the age group 65 years and older, mainly during seasons dominated by influenza A(H3N2). Older people are the most fragile in terms of the risk of dying from influenza. Despite the fact that influenza A(H3N2) dominated from week 7, excess mortality during the influenza epidemic was lower than during intense seasons dominated by influenza A(H3N2) and during 2017–2018, which was dominated by B/Yamagata.
Sentinel sampling
Virological analysis of samples from sentinel general practitioners, infectious disease clinics, and paediatric clinics contributes to national and international surveillance of circulating influenza viruses. In order to estimate what proportion of the patients seeking care for ILI actually has influenza, the different clinics are encouraged to collect nasal samples from patients with ILI. Patient characteristics, including age, sex, risk factors, syndrome (ILI vs. ARI), and vaccination status, are analysed with respect to the types of influenza that are circulating. The Public Health Agency carries out laboratory analyses for influenza free of charge for these samples. Representative positive samples are also used to characterise the circulating strains of influenza.
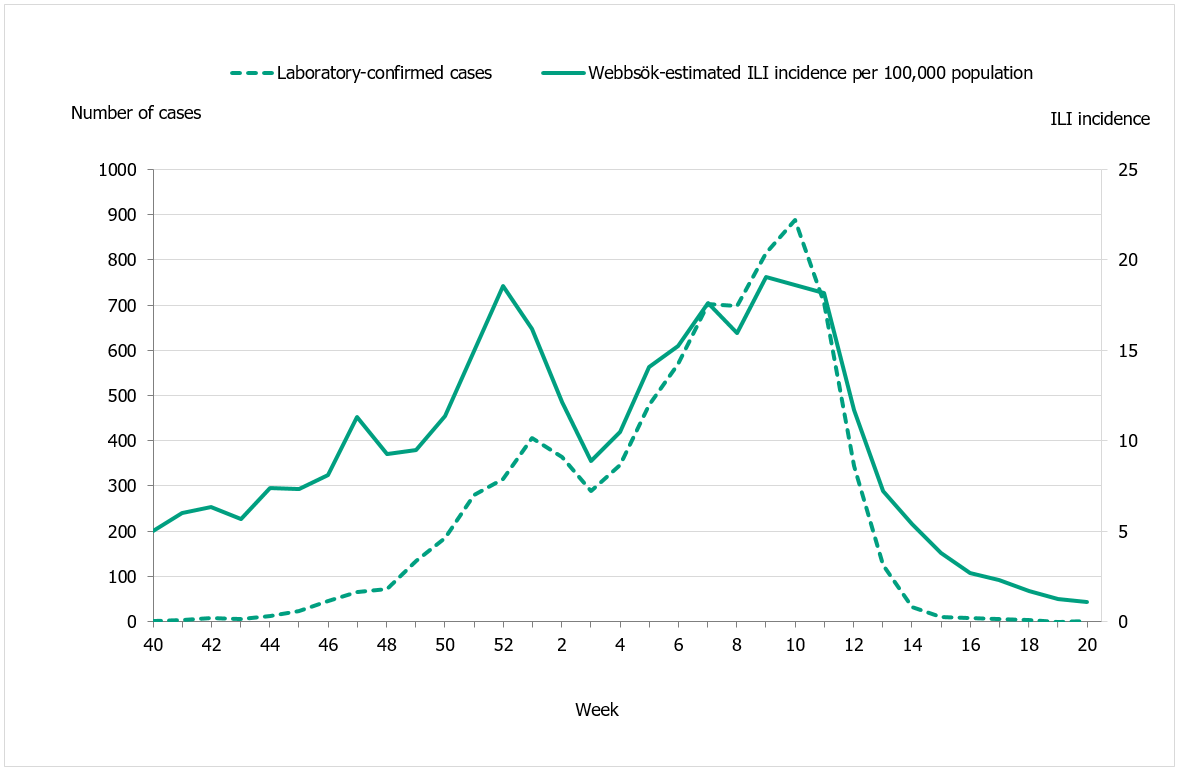
During the 2019–2020 season, 2,102 sentinel samples were submitted from 88 participants, including 84 general practitioners and 4 paediatric or infectious disease clinics. Ninety-one percent of the samples were collected by general practitioners. In total, 384 samples (18 percent) tested positive for influenza. Figure 13 shows the distribution of samples taken and the positive samples by subtype/lineage. According to the sentinel sampling surveillance system, the 2019–2020 season started in week 48, peaked in week 6, and ended in week 12.
Of the positive samples, 218 (57 percent) were positive for influenza A and 166 (43 percent) were positive for influenza B. Of the subtyped influenza A-positive samples, 125 (57 percent) were influenza A(H1N1)pdm09 and 87 (40 percent) were influenza A(H3N2). Six influenza A samples (3 percent) could not be subtyped due to low virus concentration. In total, 166 samples were positive for influenza B. Of these, all samples belonged to the B/Victoria lineage and none belonged to the B/Yamagata lineage.
As of week 10, analysis for SARS-CoV-2, the virus causing COVID-19, was included in the analysis of sentinel samples. Because of the public health measures introduced during week 11 and 12 due to the pandemic, a rapid decline in the number of cases of influenza A and B was observed. Therefore, the sentinel monitoring of influenza was shortened and terminated after week 15. Weekly reports for the sentinel sampling for COVID-19 are reported at our website (4).
Figure 13. Number of sentinel samples submitted each week, number of samples by subtype/lineage, and the percentage positive, 2019–2020.
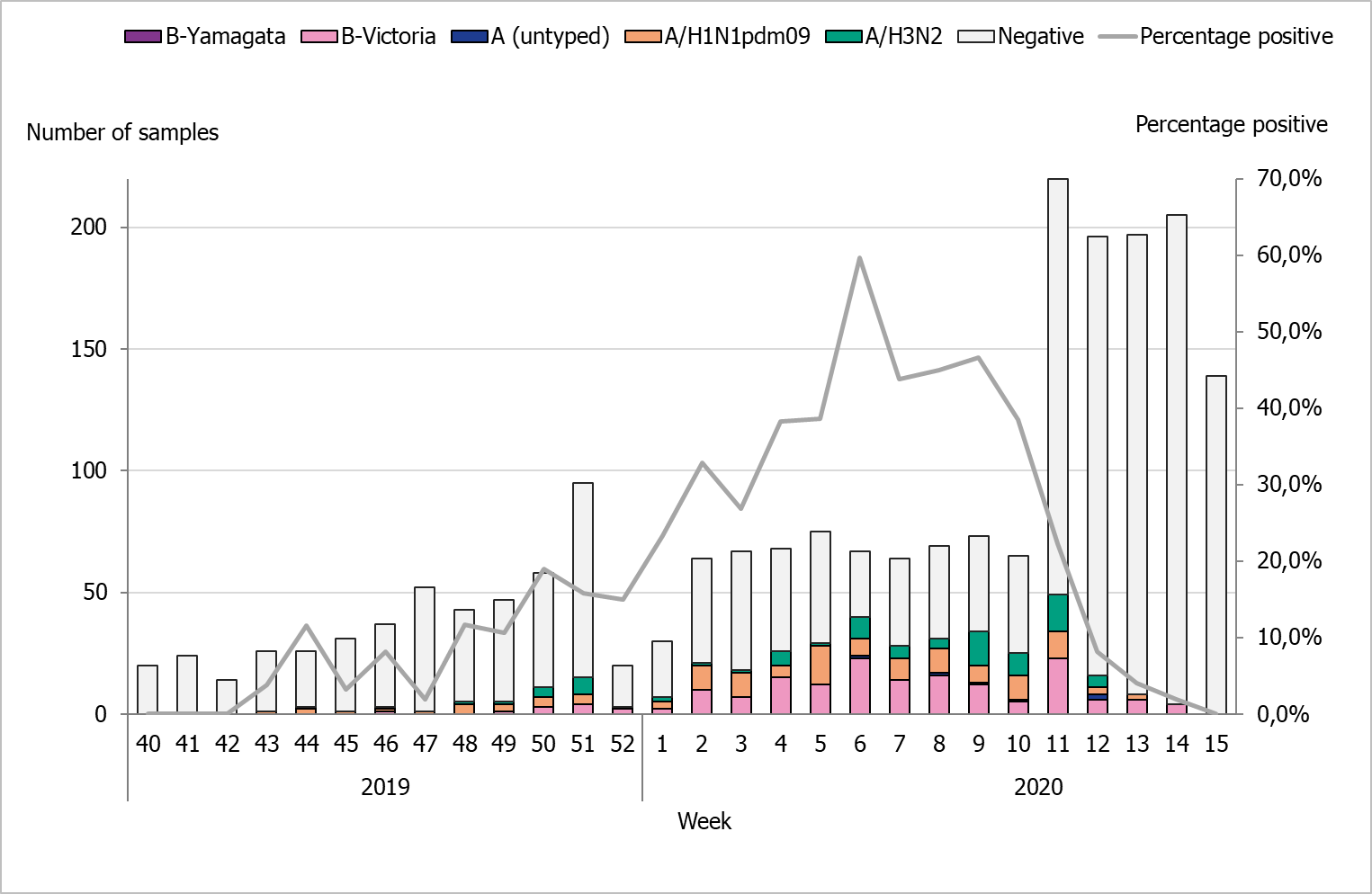
Note: As of week 10, analysis for SARS-CoV-2, the virus causing COVID-19, was included in the analysis of sentinel samples. Because of the public health measures introduced during week 11 and 12 due to the pandemic, a rapid decline in the number of cases of influenza A and B was observed. Therefore, the sentinel monitoring of influenza was shortened and terminated after week 15.
Clinical features
Of the 2,102 patients sampled through the sentinel system with known symptoms, 81 percent had ILI and 10 percent had ARI. In total, 60 percent of the samples came from women. Twenty-one percent of the samples were collected from patients belonging to a risk group due to age and/or medical condition. Of these, 60 percent were aged 65 years or older. The most common reported medical risk factors were lung disease (n = 111), heart disease (n = 62), diabetes (n = 55), immunocompromisation (n = 43), and pregnancy (n = 12). The tables below summarise the laboratory results from the sentinel sampling system for the last three seasons, including the number of samples (Table 11), median age, ILI percentage, and sub/lineage type (Table 12).
| Samples | Season 2017–2018 | Season 2018–2019 | Season 2019–2020 |
|---|---|---|---|
| Analysed | 1,616 | 1,323 | 2,102 |
| Negative | 1,075 | 915 | 1,718 |
| Positive | 541 | 408 | 384 |
| Proportion positive | 33 % | 31 % | 18 % |
| Positive for influenza A | 124 | 403 | 218 |
| Positive for influenza B | 417 | 5 | 166 |
| Samples | 2017–2018 number | 2017–2018 median age | 2017–2018 ILI % | 2018–2019 number | 2018–2019 median age | 2018–2019 ILI % | 2019–2020 number | 2019–2020 median age | 2019–2020 ILI % |
|---|---|---|---|---|---|---|---|---|---|
| Analysed | 1,616 | 42,5 | 85 | 1,323 | 41 | 92 | 2,102 | 40 | 81 |
| A(H1N1)pdm09 | 28 | 35 | 71 | 299 | 38 | 95 | 125 | 35 | 92 |
| A(H3N2) | 87 | 43 | 85 | 92 | 38 | 96 | 87 | 22 | 88 |
| A, not subtyped | 9 | 50 | 89 | 12 | 53 | 73 | 6 | 32 | 83 |
| B/Victoria | 1 | (a) | 100 | 4 | 13 | 100 | 166 | 14 | 89 |
| B/Yamagata | 415 | 41 | 89 | 1 | (a) | 100 | 0 | - | - |
| B, positive for both lineages | 1 | (a) | 100 | 0 | - | - | 0 | - | - |
(a) Median age is not shown for single cases.
Influenza infection among vaccinated patients
Vaccination status was reported for 1,898 (92 percent) of the 2,102 patients sampled during the season. Of these, 311 (16 percent) were vaccinated. Among the patients belonging to a risk group, 38 percent were vaccinated.
Influenza A was detected among 16 vaccinated patients. Influenza A(H1N1)pdm09 was detected in 9 patients (median age 58 years), influenza A(H3N2) was detected in 6 patients (median age 82 years), and one patient had non-subtyped influenza A. Nine of the 16 vaccinated patients were aged 65 years and older, and the median age for all vaccinated was 66 years. Of the seven patients younger than 65 years of age, one patient belonged to a medical risk group.
The Public Health Agency participates in the European Influenza Monitoring Vaccine Effectiveness (I-MOVE) network with data from Swedish sentinel sampling. In the interim report for the 2019–2020 season, the vaccination effect was good for A(H1N1)pdm09 (48 to 75 percent) and influenza B (62 to 83 percent), and lower for A(H3N2) (<0 to 57> percent) (1).
Subtyping and lineage determination
All diagnostic laboratories perform influenza typing using molecular assays for influenza A and B. During the 2019–2020 season, subtyping was performed at two regional laboratories (Lund and Gothenburg). The Public Health Agency performs subtyping and lineage typing by real-time PCR for all samples sent in from the diagnostic laboratories and on all positive samples from sentinel surveillance.
In total, 732 influenza A-positive samples from laboratories in Sweden were subtyped during the season, of which 264 (36 percent) were A(H1N1)pdm09) and 468 (64 percent) were A(H3N2). There was an outbreak of influenza A(H1N1)pdm09 in the northern part of the country at the beginning of the season, but thereafter a lower number of influenza A(H1N1)pdm09 cases were reported in comparison to influenza A(H3N2) based on laboratory surveillance, but both subtypes circulated. In Sweden, influenza A most recently dominated the season during 2018–2019 and 2016–2017, when influenza A(H1N1)pdm09 and A(H3N2) circulated, respectively.
The lineage was determined for 123 influenza B-positive samples. All belonged to the B/Victoria lineage and none to the B/Yamagata lineage. Typing results for subtype and lineage from sentinel and laboratory reporting systems are presented in Table 13.
In the sentinel sampling system, a higher proportion of influenza A(H1N1)pdm09 and influenza B than influenza A(H3N2) is usually detected during mixed seasons such as 2019–2020. In the sentinel system, patients are sampled by their GPs and generally have milder disease. More elderly people fall ill when influenza A(H3N2) is circulating, resulting in a higher proportion of A(H3N2) detected in the laboratory surveillance system because these samples are primarily taken at hospitals.
| Influenza type | 2015–2016, Sent. (%) | 2015–2016, Lab (%) | 2016–2017, Sent. (%) | 2016–2017, Lab (%) | 2017–2018, Sent. (%) | 2017–2018, Lab (%) | 2018–2019, Sent. (%) | 2018–2019, Lab (%) | 2019–2020, Sent. (%) | 2019–2020, Lab (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| A(H1N1)pdm09 | 71 | 63 | 1 | <1> | 6 | 13 | 76 | 63 | 33 | 25 |
| A(H3N2) | 3 | 13 | 96 | >99 | 17 | 23 | 23 | 37 | 23 | 44 |
| B/Victoria | 24 | 18 | 1 | <1> | <1> | <1> | 1 | <1> | 44 | 31 |
| B/Yamagata | 2 | 6 | 2 | <1> | 77 | 64 | <1> | <1> | 0 | 0 |
Virological analyses
A selection of the influenza-positive samples from laboratories and from the sentinel surveillance programme are further analysed for genotypic features by sequencing and for phenotypic sensitivity to neuraminidase (NA) inhibitors. Samples are selected to be as representative as possible in terms of geographical location, period of collection, and types/subtypes/lineage types. The Swedish laboratories are also asked to send influenza-positive samples from severely ill or deceased patients, patients with vaccine break-through infections, and patients who do not respond to antiviral treatment.
The main sequencing technology used is NGS (Next Generation Sequencing), which allows whole genome sequencing of all known influenza A subtypes and both influenza B lineage types. The hemagglutinin (HA) gene is characterised with respect to genetic vaccine similarity, clade affiliation, and changes in receptor affinity (lung receptors versus upper respiratory tract receptors). In addition, the HA target sequences for the subtype/lineage-specific real-time PCR systems used for detection of influenza in clinical samples are analysed for sequence mismatches compared to the real-time PCR primers and probes. The NA gene is analysed with respect to reduced or highly reduced inhibition by NA inhibitors.
In addition to NGS, Sanger sequencing of the NA gene is used for more urgent requests of genotypic antiviral resistance testing. Two aspects of the matrix protein (M) gene are analysed by sequencing, and the M2 gene of influenza A is analysed with respect to resistance to amantadine while the M target sequences of both influenza A and B of the real-time PCR systems are analysed for sequence mismatches.
Phenotypic analysis of sensitivity to the NA inhibitors oseltamivir (Tamiflu®/Ebilfumin®) and zanamivir (Relenza®) is performed with the neuraminidase inhibition (NAI) assay, which requires cell-propagated virus culture.
A representative selection of the isolated virus samples is sent to the WHO CC in London for antigenic characterisation and for phenotypic analysis of sensitivity to NA inhibitors using the NAI assay.
All characterisation data are reported to TESSy and to the Global Initiative on Sharing All Influenza Data (GISAID).
Characterisation of viruses
Genetic groups
The genetic groups of the 287 characterised Swedish influenza A and B viruses from the 2019–2020 season are shown in Tables 14A through C by influenza subtype or lineage and in the phylogenetic trees in Appendices 1–3. In total, 94, 79 and 114 samples of influenza A(H1N1)pdm09, A(H3N2) and B/Victoria were analysed, respectively. No samples of influenza B/Yamagata were received for characterisation during the season.
| Genetic group/subgroup | Number of viruses | Comment |
|---|---|---|
| 6B.1A1 | – | Vaccine virus for northern hemisphere for 2019–2020 is in this group (7). |
| 6B.1A5A | 86 (92 %) | – |
| 6B.1A5B | 7 (7 %) | – |
| 6B.1A6 | 0 | – |
| 6B.1A7 | 1 (1 %) | – |
| Genetic group/subgroup | Number of viruses | Comment |
|---|---|---|
| 3C.2a1b+T131K | 49 (62 %) | – |
| 3C.2a1b+T135K-A | 5 (6 %) | All five viruses have the A138S, G186D, D190N, F193S, and S198P amino acid substitutions. |
| 3C.2a1b+T135K-B | 3 (4 %) | – |
| 3C.3a*(Vaccine) | 22 (28 %) | Vaccine virus for northern hemisphere for 2019–2020 is in this group (7). Dominant genetic subgroup within the European surveillance system in 2019-2020 (6). |
| Genetic group/subgroup | Number of viruses | Comment | |
|---|---|---|---|
| 1A | 0 | – | |
| 1Adel162-163 | 1 (1 %) | Vaccine virus for northern hemisphere for 2019–2020 is in this group (7). | |
| 1Adel162-164A | 0 | – | |
| 1Adel162-164B | 113 (99 %) | – | |
No antigenic analyses are performed at the Public Health Agency of Sweden. The general antigenic properties of the genetic subgroups have been summarised in the report published by the WHO in conjunction with the influenza vaccine composition meeting for the northern hemisphere in February 2020 (8).
The majority of recent circulating A(H1N1)pdm09-viruses, of which most have belonged to 6B.1A, of which most (86 viruses) have been in subgroup 5A, which is also the dominant subgroup in European surveillance this season (6). In subgroup 5A, the N156K amino acid substitution is present in 26 of the Swedish viruses (30 percent). The D187A+Q189E amino acid substitutions are present in 39 of the Swedish viruses (45 percent). Viruses in 6B.1A have shown good similarity to both the cell and egg-propagated vaccine virus B/Brisbane/02/2018 in genetic subgroup 6B.1A1 according to antigenic analyses using ferret antisera. Less antigenic similarity to the current vaccine virus was seen for viruses with a substitution at amino acid 155 or 156. Results from serological assays with human sera together with the location of the amino acid substitutions indicated antigenic drift of circulating A(H1N1)pdm09 viruses including those in subgroup 6B.1A5A with D187A+Q189E.
The majority of the analysed A(H3N2) viruses in genetic subgroup 3C.3a have shown good antigenic similarity to cell-propagated vaccine virus A/Kansas/14/2017 (in subgroup 3C.3a), but less similarity to the egg-propagated vaccine virus. Viruses in genetic subgroup 3C.2a1b and subgroups within this subgroup (i.e.”T131K”,”T135K-A” and”T135K-B”) have shown less similarity to both egg and cell-propagated vaccine virus.
The majority of the analysed B/Victoria viruses belonged to subgroup 1Adel162-164B, which is also the dominant genetic subgroup within European surveillance this season (6). Viruses in subgroup 1Adel162-164 were poorly inhibited by post-infection ferret antisera raised against the current vaccine virus B/Colorado/06/2017 in subgroup 1Adel162-163.
Seven Swedish A(H1N1)pdm09 viruses and two A(H3N2) viruses collected from vaccinated individuals have been sequenced. All seven A(H1N1)pdm09 viruses belong to genetic subgroup 6B.1A5A, with four viruses also having the N156K substitution and three having the D187A+ Q189E substitution. The two A(H3N2) viruses belong to genetic subgroup 3C.2a1b+T131K and 3C.3a respectively. Five of the vaccinated individuals were 65 years or older, including the two individuals from which the A(H3N2) viruses were collected. The remaining four were 40, 44, 48, and 62 years old, all without known immunosuppression.
Antiviral sensitivity testing
The NA gene of 98 influenza A(H1H1)pdm09, 82 influenza A(H3N2), and 112 influenza B/Victoria viruses have been sequenced and analysed for amino acid substitutions known to confer reduced or highly reduced inhibition to the NA inhibitors oseltamivir and zanamivir. Three oseltamivir-resistant A(H1N1)pdm09 viruses were detected. Two of these viruses, both collected from oseltamivir-treated patients (one with known immunosuppression), carried the amino acid substitution H275Y that is known to confer clinical resistance to oseltamivir. The third patient developed symptoms and was sampled two days after finishing oseltamivir prophylaxis, and in that virus population the H275Y substitution was detected in the vast majority of the population, along with a small proportion of the N295S substitution. This latter substitution confers reduced or highly reduced inhibition to oseltamivir. None of the remaining 289 viruses carried any of the substitutions known to be associated with reduced or highly reduced inhibition to NA inhibitors. In addition, 94 A(H1N1)pdm09 viruses were analysed exclusively for the H275Y substitution by real time PCR, and none of them carried this substitution. A total of 28 viruses have been analysed for phenotypic sensitivity to NA inhibitors (oseltamivir and zanamivir) by the Public Health Agency Sweden (26 viruses) and/or WHO CC in London (12 viruses). All but one of the viruses (11 A(H1N1)pdm09, 9 A(H3N2) and 8 B/Victoria)), were shown to be sensitive to both inhibitors. The A(H1N1)pdm09 virus with amino acid substitutions H275Y (in the vast majority of the virus population)+N295S (in the minority) showed highly reduced inhibition to oseltamivir and normal inhibition to zanamivir. The amantadine resistance mutation S31N was present in all 189 analysed Swedish influenza A viruses. Two of the A(H1N1)pdm09 viruses each had one additional amantadine resistance mutation – V27A and V27I, respectively.
Virus isolation on cell culture
The majority of the samples selected for isolation are collected from other laboratories. The quality of the samples differs depending on, for example, the type of specimen, the time since sampling, and the storage and shipping conditions. Thirty-nine of the collected samples with Ct ≤ 30 were cultured on MDCK-SIAT1 cells. Five samples were excluded due to contamination with bacteria or fungi.
During the 2019–2020 season, 34 virus isolates and 34 clinical samples were shipped to the WHO CC London for further characterisation (one shipment in December and one in May).
Recommended vaccine composition
In February 2020, the WHO recommended that the quadrivalentvaccines for use in the northern hemisphere in the 2020–2021 season contain the following viruses (8).
Egg-based vaccines
The WHO recommends that the following viruses be included in egg-based vaccines:
- A/Guangdong-Maonan/SWL1536/2019, A(H1N1)pdm09-like virus (genetic subgroup 6B.1A5A +D187A, Q189E)
- A/Hong Kong/2671/2019, A(H3N2)-like virus (genetic subgroup 3C.2a1b+T135K-B)
- B/Washington/02/2019, B/Victoria lineage-like virus (genetic subgroup 1A del162-164B)
- B/Phuket/3073/2013, B/Yamagata lineage-like virus (genetic group 3)
Cell-based or recombinant-based vaccines
The WHO recommends that the following viruses be included in cell-based or recombinant-based vaccines:
- A/Hawaii/70/2019 (H1N1)pdm09-like virus (genetic subgroup 6B.1A5A (D187A, Q189E)
- A/Hong Kong/45/2019, A(H3N2)-like virus (genetic subgroup 3C.2a1b+T135K-B)
- B/Washington/02/2019, B/Victoria lineage-like virus (genetic subgroup 1A del162-164B)
- B/Phuket/3073/2013, B/Yamagata lineage-like virus (genetic group 3)
For trivalentvaccines, exclusion of the B/Phuket/3073/2013-like virus is recommended.
Vaccination coverage
Data on vaccination coverage among persons 65 years of age and older have been gathered by Sweden’s 21 county medical officers for their respective county councils since 2003. The Public Health Agency took over this task in 2014. Various methods for estimation have been used in different counties, including the use of vaccination registries, the number of vaccine doses given or distributed, sentinel reports on vaccination coverage, surveys among general practitioners, or patient record data. These methodological differences result in coverage estimates of varying quality and precision. Although the methods vary between counties, the methods within most counties have been roughly the same for the past several years, allowing a comparison over time. An estimate of the vaccination coverage in age groups younger than 65 is included, using data from a subset of county councils where registry data by age group are available throughout the season as well as annually. Data for 2019–2020 came from Gävleborg, Jämtland Härjedalen, Jönköping, Kalmar, Kronoberg, Norrbotten, Skåne, Stockholm, Sörmland, Värmland, Västernorrland, Västmanland, and Västra Götaland.
Coverage among persons 65 years of age and older
The national vaccination rate among persons aged 65 and over was 53 percent in 2019–2020, which was one percentage point higher than the previous season (see Figure 14). The Public Health Agency estimates that over 1 million people aged 65 and older were vaccinated this season. Coverage was, as in previous seasons, highest among people aged 85 and older (about 59 percent, see Table 15).
Figure 14. Vaccination coverage among persons aged 65 and older in Sweden, 2010–2011 to 2019–2020.
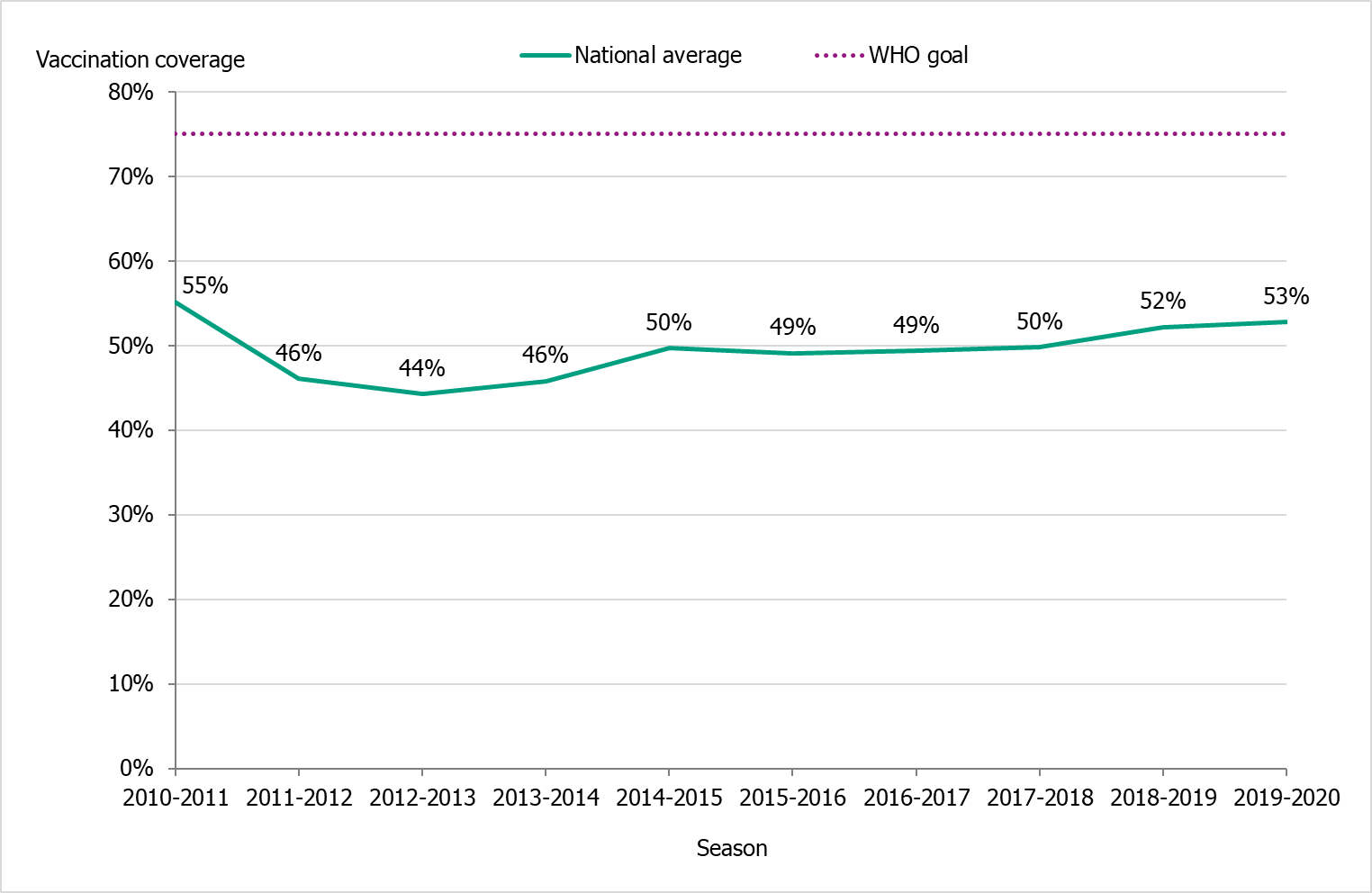
Due to delays in vaccine production, the 2019–2020 vaccination campaign started two weeks later than usual, on November 19 (week 47). The shortened period for vaccinations does not seem to have affected the coverage rate among people 65 years and older.
Regional differences in vaccination coverage
Comparisons among county councils are difficult because estimates use different methods. There is uncertainty associated with each value, but the figure below gives a picture of the current vaccination coverage rates for the age group 65 years and older throughout Sweden (see Figure 15). Different methods to estimate coverage were used in each county, which makes comparison difficult. Percentages were calculated using the county population on December 31 of each year (Source: Statistics Sweden). For more regional data and collection methods, see the Swedish-language summary of the season published in June 2020 (4).
Figure 15. Estimated proportion of vaccinated persons aged 65 and older per county council in Sweden for seasons 2018–2019 and 2019–2020.
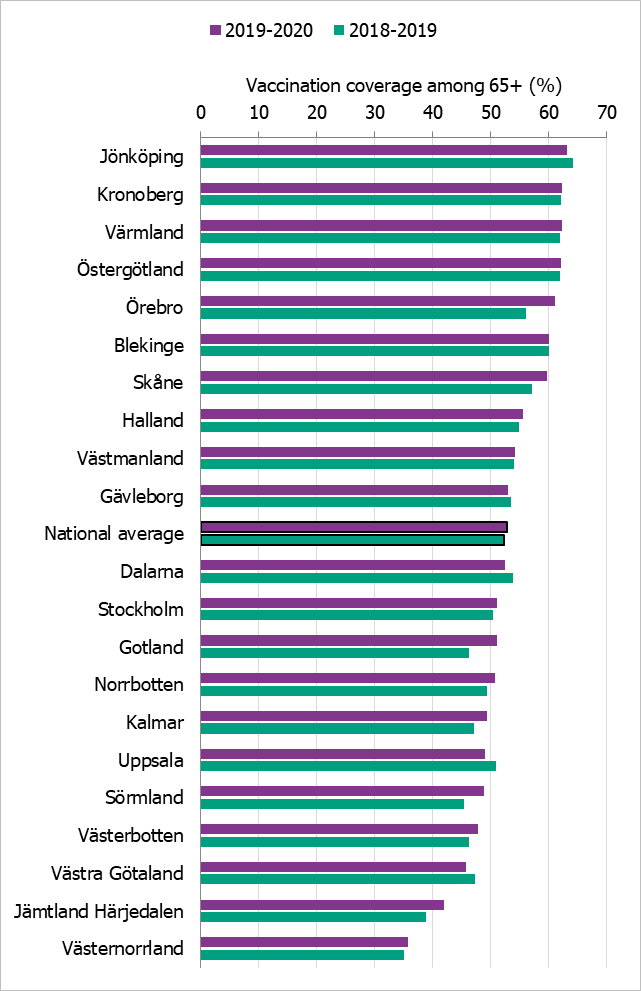
The data from Gotland come from the patient record system and directly from private healthcare offices. The data from Jämtland Härjedalen include vaccinations given within the regional healthcare system; doses given at elder care and other facilities are under-reported, which means coverage is underestimated. For Skåne, there are two estimates of the coverage each season; 2018–2019: financial system (57.1 percent), vaccination register (48.0 percent); 2019–2020: financial system (60 percent), vaccination register (49 percent). All national analyses use the financial system estimates. Data from Stockholm only include vaccinations given to people in medical risk groups (including pregnancy) or those aged 65 years or older. Uppsala data are based on reports from public and private vaccination clinics through coding of risk groups as a basis for remuneration. Data from Västernorrland only include vaccinations given within the regional health care system; doses given at elder care and other facilities are not included, which means the vaccination coverage is underestimated. Data from Västra Götaland does not include vaccinations given at private vaccination clinics. In Örebro, coverage is calculated based on data from patient records, doses given at county care facilities, and data form private vaccination clinics; doses given at hospitals are not included, which means coverage is underestimated. Data from Östergötland are based on patient records that include patients in county care facilities and the regional healthcare system, but not vaccinations given at private vaccination clinics, at pharmacies, or through occupational health services.
Vaccination coverage among persons under 65 years
It is difficult to estimate vaccination coverage among the medical risk groups under 65 years of age because these groups are hard to define and because data are often missing. Thirteen county councils have reported the number of persons vaccinated under 65 years of age, although risk group status is often unknown. See Table 15 for a breakdown by age group. Data come from Gävleborg, Jämtland Härjedalen, Jönköping, Kalmar, Kronoberg, Norrbotten, Skåne, Stockholm, Sörmland, Värmland, Västernorrland, Västmanland and Västra Götaland.
| Age group | 0–17 years | 18–39 years | 40–64 years | 65–74 years | 75–84 years | 85+ years |
|---|---|---|---|---|---|---|
| Percentage vaccinated 2018–2019 | 0.4 % | 1.8 % | 4.8 % | 44 % | 58 % | 59 % |
| Percentage vaccinated 2019–2020 | 0.4 % | 1.9 % | 4.9 % | 43 % | 58 % | 59 % |
| Population (31 Dec 2019) | 1,779,672 | 2,289,064 | 2,482,861 | 841,573 | 536,105 | 201,643 |
Syndromic surveillance
Two systems of syndromic surveillance were used during the 2019–2020 influenza season – Webbsök (Web search) and telephone calls to the medical advice line 1177 Vårdguiden. Webbsök is an automated system established in 2008 that uses a statistical model and completely anonymous data from a medical advice website to estimate the development of sentinel ILI incidence. Data are received daily and analysed weekly. The results are published on the web every Monday morning during the influenza season in the form of a graph, which is three days ahead of the publication of the weekly influenza bulletin.
In collaboration with the medical telephone advice line 1177 Vårdguiden, the Public Health Agency receives aggregated weekly data on calls from a system called Hälsoläge. The age group and main reason for calling are registered for all callers. The reported data include the number of calls related to cough, fever, and sore throat. The proportion of calls related to fever in children has been found to be a good indicator of influenza activity in the community.
Web search data (Webbsök)
According to Webbsök, the 2019–2020 influenza season lasted for 21 weeks, from week 44, 2019, to week 13, 2020 (Figure 16). The influenza activity was at low levels throughout the season. Between week 27, 2019, and week 26, 2020, a total of 334,007 queries related to influenza were entered, which was 4 percent of the total number of queries on the website 1177.se.
Webbsök had two peaks, in week 52, 2019, and weeks 9–11, 2020 and rapidly decreased from the second peak to the end of the epidemic in week 13. The second peak corresponded with the peak for the laboratory-based surveillance (Figure 17).
Figure 16. Webbsök’s estimated proportion of the population with ILI per week, 2017–2020.
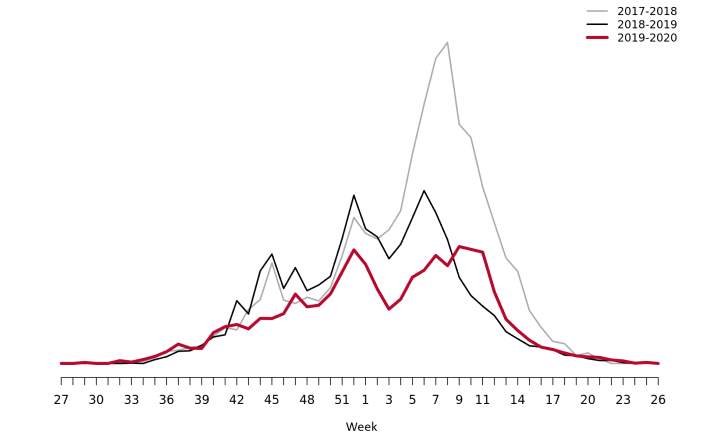
Figure 17. Webbsök’s estimated proportion of persons with ILI (incidence per 100,000 population) and the number of laboratory-confirmed cases, 2019–2020.
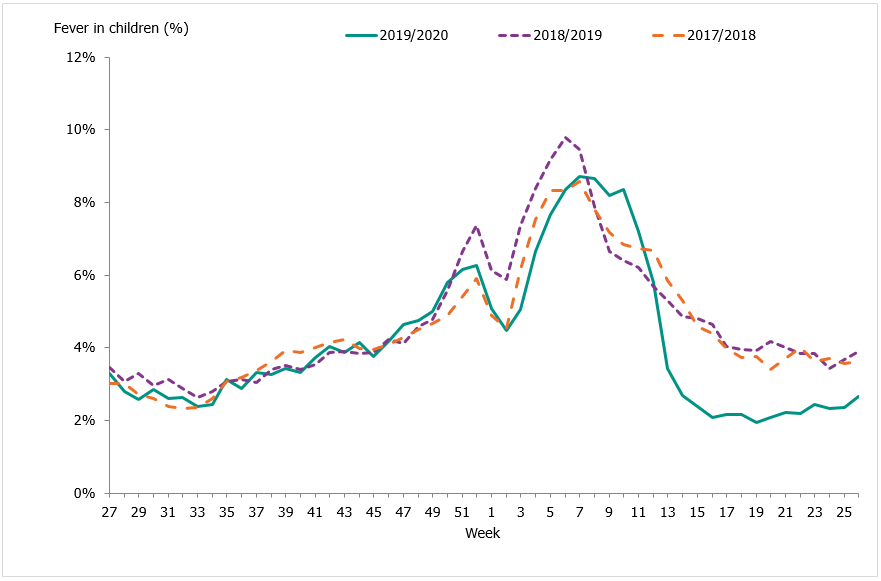
Telephone advice line data (1177 Vårdguiden)
Approximately 74,900 calls were made to the telephone advice line regarding fever in children during the season. The number of calls to 1177 Vårdguiden regarding fever in children exceeded the epidemic threshold in week 50, peaked in weeks 7–8, and ended in week 13. The percentage of calls regarding fever among children was at a low level for most of the season but reached a medium level during weeks 6–10, 2020. Like other systems, activity then rapidly decreased to the end of the epidemic in week 13.
An average of 5.0 percent of the calls throughout the season (weeks 40–20) were regarding children with fever (Figure 18). This was slightly lower than the previous season when this figure was 5.6 percent. The highest number of calls (4,205) was registered during week 10, 2020, which corresponded to the peak in the laboratory-based surveillance. The highest percentage of calls (8.7 percent) were registered during weeks 7-8, 2020. Levels this season were lower than the previous season peak (4,561 calls and 9.8 percent of all calls). A noticeable peak in calls is often seen around the Christmas holidays every year, followed by a drop. The reason for this pattern might be decreased access to face-to-face healthcare services during the holidays leading to an increase in telephone consultations.
Figure 18. Percentage of telephone calls regarding fever in children received by the medical advice line 1177 Vårdguiden for the past three seasons.
Quality assurance
Different external quality assurance programmes are used to ensure the quality of the diagnostics done in Sweden and of the analyses done by the Public Health Agency. Surveillance is dependent on standardised virological methods.
At the Public Health Agency, one-step real-time RT-PCR assays are used detect influenza A and B, to subtype the influenza A-positive samples, and to discriminate between the two influenza B lineages. These assays have also been evaluated and implemented for avian influenza diagnostics. They are sensitive, rapid, and can easily be scaled up if necessary. The Public Health Agency continuously monitors the genomic sequences of circulating influenza strains to which the PCR assays are directed in order to detect mutations that could affect their sensitivity. The Public Health Agency also performs regular validation of each assay twice a year, both ahead of the influenza season and during the peak. The PCR protocols are shared with the other laboratories in Sweden, and the laboratories that use these PCR systems are encouraged to send all samples with deviating results to the agency for sequence analysis.
Every year the Public Health Agency produces a panel PCR for the Swedish laboratories on behalf of the External Quality Assessment for Clinical Laboratory Investigations (EQUALIS). This allows the laboratories to measure the analytic sensitivity and specificity of their method. The majority of the laboratories performing diagnostics for influenza use commercial rapid PCR kits. In the beginning of the season, a questionnaire is sent to all laboratories with a question concerning methods used. Kits used by the laboratories are selected for extra quality controls during the season in order to ensure that circulating influenza strains are detected with these assays.
To ensure that the analyses performed at the Public Health Agency are correct, the agency participates in different external quality assurance programmes.
National quality assurance programme for influenza PCR
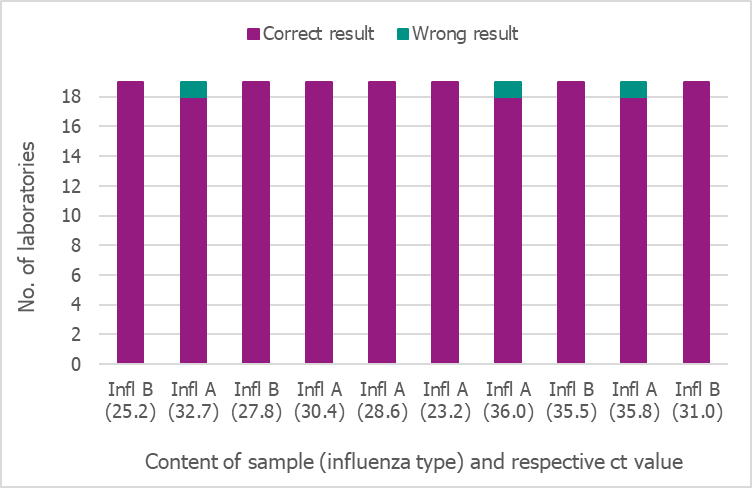
In September 2019, the Public Health Agency produced a new PCR panel for the Swedish laboratories, which was distributed by EQUALIS. Nineteen laboratories participated in the panel, and only two of them reported 8/10 and 9/10 correct results, respectively. All other laboratories reported 10/10 correct results (Figure 19).
Figure 19. Results of the Swedish External Quality Assessment panel 2019.
Control of commercial rapid PCR-kits
During the 2019–2020 season, an increasing number of laboratories used commercial rapid PCR kits for influenza diagnostics. Results of a questionnaire sent to all 25 microbiological laboratories in Sweden showed that all laboratories used a commercial PCR assay (eight laboratories used a combination of commercial and in-house assays).
Ten different kits were tested this season:
- Simplexa™ Influenza A/B & RSV Kit (Focus Diagnostics)
- GeneXpert® Xpress Flu/RSV (Cepheid)
- FILMARRAY® Respiratory Panel (Biomérieux),
- ID Now™ (Alere™) Influenza A & B Test (Abbott)
- Allplex, panel 1, 2 and 3 (Seegene)
- Accula (Mesabiotech)
- VIASURE Flu A, Flu B & RSV RT PCR Kit (CerTest Biotec)
- LightMix Modular Influenza A + Influenza B (Roche)
- Cobas Liat influenza A/B and RSV (Roche)
- FluA, FluB and RSV A/B (BioGX)
The laboratories received a number of samples on two occasions – at the beginning and during the peak of the season. A total of nine positive samples (diluted isolates) were tested with the different kits, and all gave 100 percent correct results. The results were shown on the Public Health Agency website so that other laboratories using the same kits would have knowledge of the results.
External quality assurance programmes
The Public Health Agency participated in three external quality assurance programmes during 2019–2020.
1) European External Influenza Virus Quality Assessment Programme 2020
- Results not yet available.
2) Annual WHO External Quality Assessment panel for influenza (no. 19) 2020
- Results not yet available.
3) Annual WHO External Quality Assessment panel for influenza (no. 18) 2019
- Molecular detection, typing, sub/lineage-typing.
- The 10 samples analysed by real-time PCR were correctly typed (A or B), and 9/10 were correctly subtyped. One sample (type A) could not be subtyped with this technique but was identified as subtype A(H9) by WGS (NGS) and later confirmed by the final report. This result was expected because the Public Health Agency does not have a real-time PCR system for H9 subtyping.
- Genotypic and phenotypic antiviral susceptibility determination.
- Genotypic analysis was performed by NGS (Ion Torrent), and the results were correct for all four samples.
- Phenotypic analysis (fluorescence-based MUNANA) could not be completed by the 2019-06-14 deadline due to the purchase of new fluorometer. The four samples were, however, analysed retrospectively (completed 2019-07-05) and the results were correct compared to the expected results.
3) INFRNA panel from Quality Control for Molecular Diagnostics (QCMD) 2019
- Typing, sub/lineage-typing. All 10 samples were correctly typed/subtyped by real time PCR.
References
- Angela Rose, Esther Kissling, Hanne-Dorthe Emborg, Amparo Larrauri, Jim McMenamin, Francisco Pozo, Ramona Trebbien, Clara Mazagatos, Heather Whitaker, Marta Valenciano, European IVE group. Interim 2019/20 influenza vaccine effectiveness: six European studies, September 2019 to January 2020.Euro Surveill. 2020;Mar;25(10):2000153. https://doi.org/10.2807/1560-7917.es.2020.25.10.2000153
- Public Health Agency of Sweden. Publikationer [Publications]. [Internet]. Stockholm: Public Health Agency of Sweden; 2020 [cited 2020-09-03] Available from: http://www.folkhalsomyndigheten.se/publicerat-material/publikationer/
- Public Health Agency of Sweden, Aktuell influensarapport [Current Weekly Report]. [Internet]. Stockholm: Public Health Agency of Sweden; 2020 [cited 2020-08-27] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/influensa-veckorapporter/arkiv-20192020/
- Public Health Agency of Sweden, Aktuell veckorapport om covid-19 [Current Weekly COVID-19 Report]. [Internet]. Stockholm: Public Health Agency of Sweden; 2020 [cited 2020-09-08] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/covid-19-veckorapporter/senaste-covidrapporten/
- Public Health Agency of Sweden, Sammanfattande rapport för säsongen 2019–2020 [Summary report for the 2019–2020 season]. [Internet] Stockholm: Public Health Agency of Sweden; 2020 [cited 2020-09-03] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/influensa-veckorapporter/arkiv-20192020/
- European Centre for Disease Prevention and Control/World Health Organisation Regional Office for Europe. Flu News Europe, Joint ECDC–WHO weekly influenza update, week 20/2020. [Published on 2020-05-26, cited 2020-09-08]. Available from: se http://flunewseurope.org/Archives
- World Health Organization, Addendum to the recommended composition of influenza virus vaccines for use in the 2019–2020 northern hemisphere influenza season. [Internet]. Geneva: World Health Organization; 2019. [published 2019-02-21, updated 2019-03-21, cited 2019-08-08]. Available from: https://www.who.int/influenza/vaccines/virus/recommendations/2019_20_north/en/
- World Health Organization, Recommended composition of influenza virus vaccines for use in the 2020–2021 northern hemisphere influenza season. [Internet]. Geneva: World Health Organization; 2020. [published 2019-02-28, cited 2020-08-27]. Available from: https://www.who.int/influenza/vaccines/virus/recommendations/2020-21_north/en//
- Public Health Agency of Sweden, Pandemiberedskap [Pandemic preparedness]. [Internet] Stockholm: Public Health Agency of Sweden; 2019 [cited 2020-09-03] Available from: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/krisberedskap/pandemiberedskap/
