
Influenza in Sweden – Season 2017–2018
Summary
The 2017–2018 influenza season was dominated by influenza B/Yamagata with a subsequent smaller wave of influenza A. It is unusual for a season to be dominated by influenza B. In fact, this has only occurred two times during the previous 21 seasons in Sweden.
The season's epidemic started in week 49 (early December) and reached its peak in week 7 (mid-February). In total, 20,686 laboratory-confirmed influenza cases were reported, 64 percent of which were influenza B. Compared to the previous seasons, significantly more influenza cases were reported, and the number of analysed samples (a total of 88,837 samples) increased, mainly from outpatient care. Faster and more accessible diagnostics have probably contributed to the large increase in the number of analysed samples.
Telephone calls to the medical advice line 1177 and web search surveillance showed the season to be intense with many sick children and adults. Influenza B/Yamagata is known to infect many children and young adults when it circulates in the community, but these age groups often recover from influenza illness at home and do not need medical care. Therefore, this age profile is not reflected in the age distribution of the laboratory-confirmed influenza B cases, which consists of those who have sought health care. The majority of these were 65 years of age and older. Similarly, the number of cases per population (incidence) in each age group was highest among those 65 years and older, with 359 cases per 100,000 individuals. From week 12 onward, virus dominance switched from influenza B to A. Of subtyped influenza A samples, 65 percent were A(H3N2). In total, 58 percent of the influenza A cases were diagnosed among people aged 65 and older. The incidence was also highest in this age group (211 cases per 100,000).
The average vaccination coverage among people 65 years and older was 49 percent, which is the same level as recent seasons. Vaccination coverage is collected in different ways, and small differences from year to year can occur. The coverage rate is highest among people 75 years and older (over 55 percent). This is positive because the risk of severe influenza illness increases with increasing age. Among people under the age of 65, it is estimated that 5–10 percent belong to a risk group, but the vaccination coverage in this age group remains at only 2 percent.
The use of antiviral medication for the treatment of severe influenza disease and prophylaxis increased greatly this season following the increase in laboratory-confirmed influenza cases. Prescriptions increased by 53 percent and requisitions from healthcare increased by 29 percent.
During the season, 447 patients were reported to be in intensive care with influenza. Of these, 15 patients required extracorporeal membrane oxygenation (ECMO) treatment, and 9 of these patients were under the age of 40. Of the intensive care patients, 288 had influenza B and 159 had influenza A. Of all intensive care patients, 74 percent belonged to at least one medical risk group or were 65 years of age or older. Among patients under the age of 65, over half (116 patients, 54 percent) did not belong to a medical risk group. Of those who belonged to a risk group and needed intensive care during this season, only 23 percent of those with known vaccination status were vaccinated.
Influenza can cause or contribute to deaths among all age groups, but monitoring shows that deaths occur mainly in the older age groups. During the season, significant influenza-related excess mortality was seen among people 65 years and older during weeks 3 to 15. The level of excess mortality was comparable to that for the 2016–2017 season when the age group 65 years and older was severely affected by influenza A(H3N2). It is unusual that a season dominated by influenza B causes such high excess mortality because influenza B does not usually cause major epidemics, but it confirms that the elderly are the most vulnerable age group in terms of the risk of dying from influenza.
Among those who received a laboratory-confirmed influenza diagnosis, 4.9 percent died within 30 days of the laboratory diagnosis, which was similar to the previous season when 5.6 percent died. In the analysis of the 1,021 deaths that occurred within 30 days of diagnosis, 93 percent of the deaths were in the age group 65 years and older. The proportion of laboratory cases that died increased with increasing age.
Characterisation of the viral strains collected through sentinel sampling and from laboratories around Sweden showed antigenic similarity to the A(H1N1)pdm09 vaccine strain. The majority of the A(H3N2) strains showed antigenic similarity to cell-cultured but poor similarity to egg-cultured vaccine strains. The coming season, the A(H3N2) component of the vaccine will be changed to address this problem. B/Yamagata strains belonged to the genetic group that is antigenically similar to the vaccine strain in the tetravalent vaccine (but not included in the trivalent vaccine). In the global surveillance system, viruses with a deletion of HA1 amino acids 162-163 have reacted poorly to B/Brisbane/60/2008 in antigenic analyses, which led the WHO to change the B/Victoria strain for the 2018–2019 season vaccine in the northern hemisphere. Only one of 347 analysed strains was resistant to oseltamivir (but none was resistant to zanamivir), and this was an A(H1N1)pdm09-virus.
In sentinel surveillance, most cases in the 2017–2018 season were B/Yamagata (76.9 percent), followed by A(H3N2) at 16.5 percent and A(H1N1)pdm09 at 5.4 percent. The Public Health Agency participates in the European Influenza Monitoring Vaccine Effectiveness (I-MOVE) network with data from Swedish sentinel sampling. In the interim report for the 2017–2018 season, the vaccine effectiveness against A(H1N1)pdm09 was good (55–68 percent), the effectiveness against B was moderate (36–54 percent), and the effectiveness against A(H3N2) was low (≤7 percent). This showed that the B/Victoria strain in the trivalent seasonal influenza vaccine gave some cross-protection against B/Yamagata, the virus circulating during the season.
Sammanfattning
Influensasäsongen 2017–2018 dominerades av influensa B/Yamagata med en efterföljande våg av influensa A. Det är ovanligt att en säsong domineras av influensa B, och det har bara hänt under 2 av de föregående 21 säsongerna.
Säsongens epidemi startade vecka 49 och nådde sin topp vecka 7. Totalt rapporterades 20 686 laboratorieverifierade influensafall, varav 64 procent var influensa B. Jämfört med de föregående säsongerna rapporterades betydligt fler influensafall och antalet analyserade prover (totalt 88 837 prov) ökade, främst från öppenvården. Snabbare och mer tillgänglig diagnostik har troligtvis bidragit till den stora ökningen. Även antalet webbsökningar och telefonsamtal till 1177 Vårdguiden visade på en intensiv säsong med många sjuka barn och vuxna.
Influensa B/Yamagata är sedan tidigare känd för att främst drabba barn och unga vuxna när det cirkulerar i samhället, men personer i dessa åldersgrupper klarar ofta av att tillfriskna från sin influensa hemma och behöver inte uppsöka sjukvård. Därför är det inte yngre åldersgrupper som dominerar bland de laboratorieverifierade fallen, det vill säga de personer som sökte vård. De flesta laboratorieverifierade fallen av influensa B återfinns i stället bland personer 65 år och äldre. Den gruppen var hårdast drabbad sett till incidensen, alltså antalet fall i en viss befolkningsgrupp, med en incidens på 359 fall per 100 000 invånare. Vecka 12 skiftade dominansen från influensa B till A, och så fortsatte resten av säsongen. Av de subtypade influensa A-fallen var 65 procent A(H3N2). Även bland de fallen drabbades främst personer 65 år och äldre, med 58 procent av de laboratorieverifierade fallen. Incidensen var också högst i denna åldersgrupp med 211 fall per 100 000 invånare.
Vaccinationstäckningen bland personer 65 år och äldre var i genomsnitt 49 procent och har legat på samma nivå under de senaste säsongerna. Uppgifter om vaccinationstäckningen samlas dock in på olika sätt och små skillnader från år till år kan uppstå. Täckningsgraden är som högst bland personer 75 år och äldre (drygt 55 procent). Detta är positivt eftersom stigande ålder ökar risken för svår sjukdom vid influensainfektion. Bland personer under 65 år uppskattas att 5–10 procent tillhör en riskgrupp, men vaccinationstäckningen i denna åldersgrupp är endast 2 procent.
Det finns antiviralt läkemedel för behandling av svår influensasjukdom samt profylax, och försäljningsmönstret i Sverige följer laboratoriefallen. Denna säsong ökade antalet uthämtade recept med drygt 50 procent jämfört med föregående säsong, medan rekvisitioner från vården ökade med cirka 30 procent.
Under säsongen var det 447 patienter i landet som rapporterades som intensivvårdade med influensa. Av dem behövde 15 patienter vård i ECMO (extrakorporeal membranoxygenering), och 9 av dessa patienter var under 40 år. Av de intensivvårdade patienterna hade majoriteten influensa B, 288 stycken, medan 159 patienter hade influensa A. Av alla intensivvårdade patienter tillhörde 74 procent minst en medicinsk riskgrupp eller var 65 år och äldre. Bland patienter under 65 år tillhörde över hälften (116 patienter, motsvarande 54 procent) inte en medicinsk riskgrupp. Av de som tillhörde en riskgrupp och behövde intensivvård under denna säsong var endast 23 procent vaccinerade, sett till dem med känd vaccinationsstatus.
Influensa kan bidra till dödsfall i alla åldersgrupper, men övervakningen visar att huvudsakligen äldre drabbas. Denna säsong uppmättes en influensarelaterad överdödlighet bland personer 65 år och äldre under vecka 3–15. Överdödligheten motsvarar den för säsongen 2016–2017, då samma åldersgrupp drabbades hårt av influensa A(H3N2). Det är dock ovanligt att en säsong som domineras av influensa B orsakar så hög överdödlighet eftersom den typen inte brukar orsaka så stora epidemier, men detta visar att äldre personer löper störst risk att dö av influensa. Bland de personer som fick en laboratorieverifierad influensadiagnos hade 4,9 procent dött inom 30 dagar, vilket liknar den föregående säsongen då andelen var 5,6 procent. Totalt 1 012 dödsfall skedde inom 30 dagar, och 93 procent gällde personer i åldersgruppen 65 år och äldre. Andelen dödsfall ökade med stigande ålder.
Viruskarakteriseringen sker på ett urval av de stammar som samlats in genom sentinelprovtagningen och från laboratorier i landet. Alla analyserade stammar visar antigenisk likhet med vaccinstammen för A(H1N1)pdm09. Majoriteten av A(H3N2)- stammarna hade antigenisk likhet med cellodlad vaccinstam men sämre likhet med äggodlad vaccinstam. Av denna anledning har A(H3N2)-stammen i vaccinet bytts inför kommande säsong. Influensa B/Yamagata-stammarna tillhörde en genetisk grupp som är antigeniskt lik vaccinstammen i det fyrvalenta vaccinet (ingår inte i det trivalenta vaccinet). Endast 1 stam av 347 analyserade var resistent mot oseltamivir (inte mot zanamivir).
Inom sentinelprovtagning var 76,9 procent av proverna B/Yamagata, följt av 16,5 procent A(H3N2) och 5,4 procent A(H1N1)pdm09. Folkhälsomyndigheten deltar i det europeiska nätverket för att mäta influensavaccinets effekt (I-MOVE) med hjälp av data från den svenska sentinelprovtagningen. Enligt interimsrapporten för säsongen 2017–2018 var vaccinationseffekten för A(H1N1)pdm09 god (55–68 procent), för B måttlig (36–54 procent) och för A(H3N2) låg (< 7 procent). Detta visade att B/Victoria i det trivalenta säsongsinfluensavaccinet gav korsskydd mot B/Yamagata, den linjetyp som cirkulerade under säsongen.
About this publication
This report describes the monitoring systems for influenza in use during the winter season of 2017–2018 and the results of both epidemiological and virological surveillance. Data are also compared to previous influenza seasons.
The report has been prepared for the World Health Organization (WHO) as part of the Public Health Agency of Sweden’s function as a National Influenza Centre (NIC).
Annual reports in English about the influenza seasons in Sweden have been available since 1997, and those from 2000–2001 onward can be found on the Public Health Agency’s website (suggested search “Influenza in Sweden”) (1).
The report has been prepared by Mia Brytting, Helena Dahl, and Åsa Wiman of the Unit for Laboratory Surveillance of Vaccine Preventable Diseases and by Emma Byström, AnnaSara Carnahan, and Marie Rapp of the Unit for Vaccination Programs.
Public Health Agency of Sweden
Tiia Lepp
Head of Unit
Unit for Vaccination
Mia Brytting
Head of Unit
Programs Unit for Laboratory Surveillance of Viral Pathogens and Vaccine Preventable Diseases
Influenza surveillance in Sweden
Influenza epidemics recur in Sweden each winter, with a range of effects depending on the characteristics of the circulating viruses and the level of immunity in different age groups. New influenza strains, particularly those different enough to cause a pandemic, can be very aggressive and can cause severe illness, and these can cause great strain on intensive care units and can lead to deaths in all age groups. None of these effects are detectable through a single reporting system. In order to get an overall picture of on-going influenza activity and to remain prepared in case of a pandemic, the Public Health Agency of Sweden (Folkhälsomyndigheten) has a number of different epidemiological reporting systems for influenza ranging from the collection of data from different healthcare providers to the analysis of web searches.
Virological surveillance is as important as epidemiological reporting systems. Viruses are typed as influenza A or B by regional laboratories in real time during the influenza season, and some laboratories also determine the subtype for influenza A. Throughout the season, viruses from around the country are characterised by the Public Health Agency with regard to subtype and lineage, vaccine similarity, sensitivity to antiviral drugs, and other factors that might affect the severity of the infections they cause. Viruses are also isolated and sent to the WHO Collaborating Centre (WHO CC) in London for further characterisation and to provide a basis for vaccine strain selection. When new strains of influenza virus emerge, reference methods for diagnostics are established at the Public Health Agency and shared with all microbiological laboratories in Sweden.
During the influenza season, the Public Health Agency condenses national and international data into a detailed weekly bulletin that is published on the agency’s website (2). A preliminary summary of the 2017–2018 season was published on June 12, 2018 (3). The bulletin provides timely analysis of the current situation in Sweden and abroad and has a wide readership.
Vaccine strains
Vaccine strains 2017–2018
At the end of February 2017, the WHO recommended that the trivalent vaccines for use during the 2017–2018 northern hemisphere influenza season contain the following (4):
- An A/Michigan/45/2015 (H1N1)pdm09-like virus
- An A/Hong Kong/4801/2014 (H3N2)-like virus
- A B/Brisbane/60/2008-like virus (B/Victoria/2/87 lineage).
The WHO recommended that the quadrivalent vaccines contain, in addition to the viruses listed above, a B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage).
Vaccine strains 2018–2019
At the end of February 2018, the WHO recommended that the A(H3N2) and B/Victoria strains be changed in the trivalent vaccines for use in the 2018–2019 northern hemisphere influenza season (5).
The recommended content of the trivalent vaccines for the 2018–2019 season is:
- an A/Michigan/45/2015 (H1N1)pdm09-like virus
- an A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- a B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage)
The WHO recommended that the quadrivalent vaccines contain, in addition to the viruses listed above, a B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage).
Updates to National policies and recommendations
During the 2017–2018 season and the summer of 2018, the following policies and recommendations were updated:
- Updated vaccination recommendations, March and September 2018 (6)
- Updated web-based information about vaccination and influenza
- New Pandemic Vaccination Plan, published September 2017 (7).
Work is on-going on updates to other pandemic preparedness documents, including a new National Pandemic Strategy, a background document, and other related documents. The latest documents can be found on the Public Health Agency website under Pandemic Preparedness (8).
Surveillance 2017–2018
The pyramid below illustrates the different ways that influenza affects those who are infected (Figure 1), ranging from the large number of those infected to the small number who die as a result of the influenza infection. Table 1 describes the data collection systems that were used to monitor influenza activity at the various levels of the influenza pyramid in Sweden during the 2017–2018 season, and it summarises the results of each system. Each system is described further at the start of each section below showing the season’s results.
Table 2 shows which week each system, as applicable, crossed the threshold for epidemic start, reached its peak notation, and crossed the threshold for the end of the epidemic, as well as the maximum intensity level measured by the system during the season.
Figure 1. The “influenza pyramid” showing possible outcomes of an influenza infection and the surveillance systems at each level.
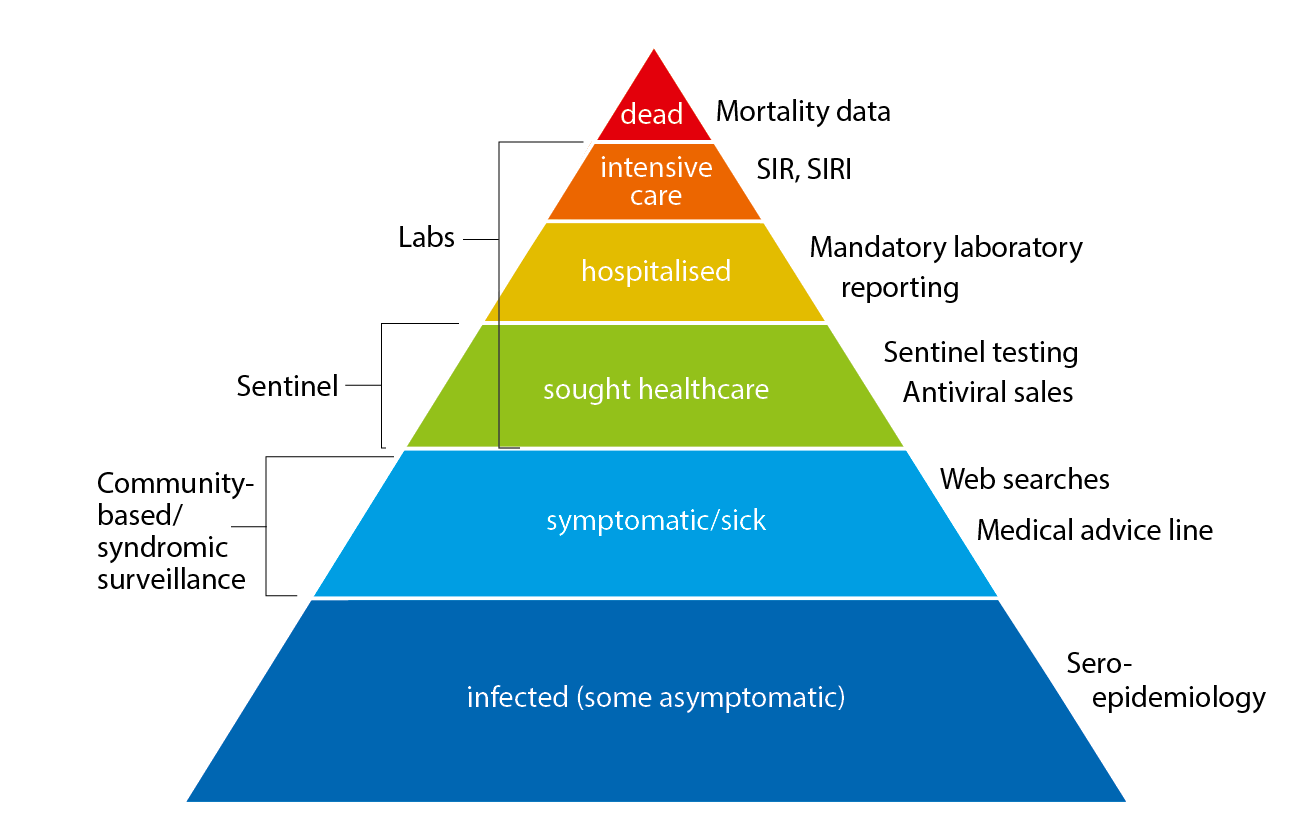
SIR: Swedish Intensive Care Registry
SIRI: Swedish Intensive Care Registry – Influenza module
| Reportingsystem/method | Implementation | What does the system/method show? | Results (2017–2018) | ||
|---|---|---|---|---|---|
| Statutory laboratory reporting of influenzaVoluntary reporting of denominator data and sub-/lineage typing results | Legal obligation for all laboratories to report influenza diagnoses along with full patient identity in the web-based reporting system, SmiNet, in accordance with the Communicable Diseases Act. | Number of laboratory-confirmed cases of influenza A and B together with age, gender, and geographical distribution.Proportion of samples tested that are positive for an influenza virus and sub-/lineage type | 13,280 laboratory-confirmed cases of influenza A and 7,406 cases of influenza B. 88,837 samples, 23.3% positive: 36% influenza A 64% influenza B Of influenza A cases subtyped: 65% A(H3N2) 35% A(H1N1)pdm09 Of influenza B cases with determined lineage: >99% B/Yamagata <1% b victoria> |
||
| Antiviral sales | Weekly data from the Swedish eHealth Agency | Number of packages sold by type of sale, including prescriptions and health care requisitions. | 20,566 packages | ||
| Voluntary clinical reporting of laboratory-confirmed influenza cases (all types) in intensive care (SIRI) | Collaboration with the Swedish Intensive Care Registry (SIR). Treating physicians in intensive care units are asked to report clinical information about patients with laboratory-confirmed influenza. | Severity of infections with different influenza subtypes and impact on the intensive care units. | 447 laboratory-confirmed cases of influenza were reported from SIRI. Of those, 137 were reported as influenza A (unknown subtype), 9 were A(H1N1)pdm09, 13 were A(H3N2), and 288 were influenza B. |
||
| Excess mortality | Weekly data on the aggregated number of deaths in Sweden, by age group, is sent from the Swedish Tax Agency to the Public Health Agency and analysed with statistical models. | Influenza-attributable excess mortality (FluMoMo model)All-cause mortality (EuroMoMo model) | Significant influenza-attributable excess mortality was seen (FluMoMo) among persons aged 65 years and older in weeks 3–15, 2018. | ||
| Deaths within 30 days | Weekly data on date of death are sent from the Swedish Tax Agency to the Public Health Agency and analysed intermittently. | Death within 30 days of influenza diagnosis | 1,021 of 20,857 persons with an influenza diagnosis had died within 30 days of diagnosis, of which 67% were influenza B. Most (93%) were aged 65 years and older. | ||
| Sentinelsampling | Samples from some patients who present with influenza-like illness (ILI), as well as some patients with acute respiratory illness (ARI), are analysed by the Public Health Agency for influenza. | The proportion of sentinel patients with ILI or ARI who have an influenza infection (see also virus characterisation below). | 1,616 samples were analysed, of which 541 (33.5%) tested positive for influenza: 77% B/Yamagata-like | ||
| Viruscharacterisation | Continual genotypic and phenotypic assays of laboratory and sentinel samples that tested positive for influenza. | Viruses' clade affiliation and genetic vaccine similarity, and possible resistance to antiviral drugs. | Genetic characterisation The 123 analysed B/Yamagata viruses belonged to genetic clade 3. The majority of circulating viruses in this clade react well against ferret antisera raised against both cell- and egg- propagated vaccine virus (5). 63 of 104 A(H3N2) viruses belonged to genetic clade 3C.2a, 38 belonged to subclade 3C.2a1, and 3 belonged to clade 3C.3a. Clade 3C.2a and subclade 3C2a.1 viruses have been shown to have antigenic properties similar to the cell- but not the egg-propagated vaccine virus (5). All 48 A(H1N1)pdm09 viruses belonged to group 6B, a subgroup in which the majority of the viruses have antigenic properties similar to the vaccine virus (5). No B/Victoria viruses have been characterised due to lack of samples with a sufficient amount of viral RNA. Analysis of the neuraminidase gene with respect to mutations associated with reduced or highly reduced inhibition to neuraminidase inhibitors: 347 viruses analysed, including 72 A(H1N1)pdm09 viruses analysed exclusively for the H275Y mutation. One A(H1N1)pdm09 virus had the H275Y mutation, which is associated with highly reduced inhibition to oseltamivir but not to zanamivir. The remaining 346 viruses did not harbour any mutations. Phenotypical analysis for resistance to oseltamivir and zanamivir: 28 viruses analysed All tested viruses were sensitive to both oseltamivir and zanamivir. |
||
| Vaccination coverage | Periodic collection of coverage data from county councils. | Vaccination coverage per age group. | 49.4% coverage among those 65 years or older ~2% coverage among risk groups under 65 years |
||
| Webbsök (Web Search) | An automated system that uses search data from the national medical advice site 1177.se. The numbers of searches on influenza and influenza symptoms are entered into a statistical model that estimates the proportion of patients with ILI visiting general practitioners. | Estimates the proportion of patients with ILI. | Between week 27, 2017, and week 26, 2018, a total of 394,960 queries related to influenza were entered, which was 4.7% of the total number of queries on the website 1177.se. Webbsök’s influenza season lasted for 25 weeks (week 43, 2017–week 15, 2018), with weeks 5–9 at high or very high level. |
||
| Telephone Advice Line (1177 Vårdguiden) | Weekly aggregated data on the primary reason for contacting the medical advice line (phone number 1177) and the age group of the person concerned are manually reported to the Public Health Agency through the system Hälsoläge. Data are collected from 18 of Sweden’s 21 county councils (Stockholm, Sörmland, and Värmland are excluded because of data irregularities). | Primary reason for calling by age group (adults and children). | Approximately 107,500 calls regarding fever in children. Fever in children accounted for 5.4% of all calls to 1177 during the year. The epidemic started in week 52, 2017, and the peak was week 7, 2018, with 8.6% of calls due to fever in children. The epidemic ended in week 17. |
||
| System | Start | Peak | End | Max intensity |
|---|---|---|---|---|
| Laboratory-based surveillance (number of cases) | 49 | 7 | 17 | Very high |
| Laboratory-based surveillance (% positive) | 49 | 7 | 17 | Medium |
| Antiviral sales | * | 7 | * | * |
| Laboratory-confirmed influenza cases in intensive care (SIRI) | * | 5–8 | * | * |
| Excess mortality | 3 | 8 | 15 | * |
| Sentinel sampling | 50 | 6 | 16 | * |
| Webbsök (Web Search) | 43 | 8 | 15 | Very high |
| Telephone Advice Line (1177 Vårdguiden) | 51 | 5–7 | 17 | Medium |
* Epidemic thresholds/intensity levels not assigned.
Laboratory-based surveillance
All laboratory-confirmed cases of influenza fall under statutory reporting requirements (as of Dec 1, 2015), but subtyping is not required. Denominator data (the total number of samples analysed) are reported voluntarily via e-mail. Sampling for influenza in Sweden is primarily done in hospital settings.
The influenza season 2017–2018 was dominated by influenza B followed by a wave of influenza A (see Figure 2). The epidemic started in week 49 (early December) and continued for 21 weeks until it ended in week 17. After the start of the epidemic, the number of laboratory-confirmed influenza cases increased throughout the month of December and then reached a plateau. A sharp increase in the number of cases was seen in in week 3 and onwards until the peak in week 7, when 2,159 cases were reported (see Figure 3). Decreased or plateaued influenza activity during the Christmas and New Year holidays has been observed in the majority of previous seasons. The last time influenza B dominated the influenza season was in 2010–2011, during which influenza B/Victoria circulated.
The majority of the cases (>78%) during the peak (week 7) were influenza B, and based on the samples whose lineage was determined, influenza B/Yamagata was the dominating lineage throughout the season. Of the cases reported for weeks 40–20, 64% of the cases were influenza B and 36% were influenza A. At the end of January, influenza A started to increase slowly until it peaked in week 12, at which point dominance also shifted from influenza B to A. Of the samples subtyped, influenza A(H3N2) accounted for the majority of the samples (65 percent).
In comparison with previous seasons, there was a substantial increase in reported laboratory-confirmed influenza cases. In total, 20,686 cases were reported throughout the season. During the peak week (week 7), the weekly number of cases (2,159) was higher than any previous season peak (see Figure 4).
The number of individuals who had a sample taken for influenza diagnosis also increased compared to previous seasons. More than 88,000 samples were taken in 2017–2018, and 23.3% of these samples were positive for influenza (Table 3). Compared to previous seasons, the overall percentage that were positive indicates that slightly more patients who were sampled had influenza than the previous two seasons, but this was still less than the proportion that were positive in the intense season 2014–2015. Compared to 2014–2015, the peak percentage positive was higher in 2017–2018 (38.7 percent), but the peak was narrower (Figure 4).
An analysis of the level of care at sampling for positive samples over the past three seasons showed that the greatest increases were in samples taken in inpatient care (60% increase from the last season) and outpatient care (76% increase), whereas samples taken in emergency care only increased slightly (Figure 5). Faster and better availability of virological analysis of samples has most likely contributed to the substantial increase of both sampling and reported cases from outpatient care clinics. Similarly, diagnostics have also been implemented at hospitals that do not have microbiological laboratories during the last few years, and this change has facilitated fast analysis for influenza in more hospitals than ever before. In addition, the increased availability of fast diagnostics enables hospitals to run analysis around the clock, seven days a week.
Figure 2. Number of laboratory-confirmed cases by influenza type and week, 2016–2017 and 2017–2018.
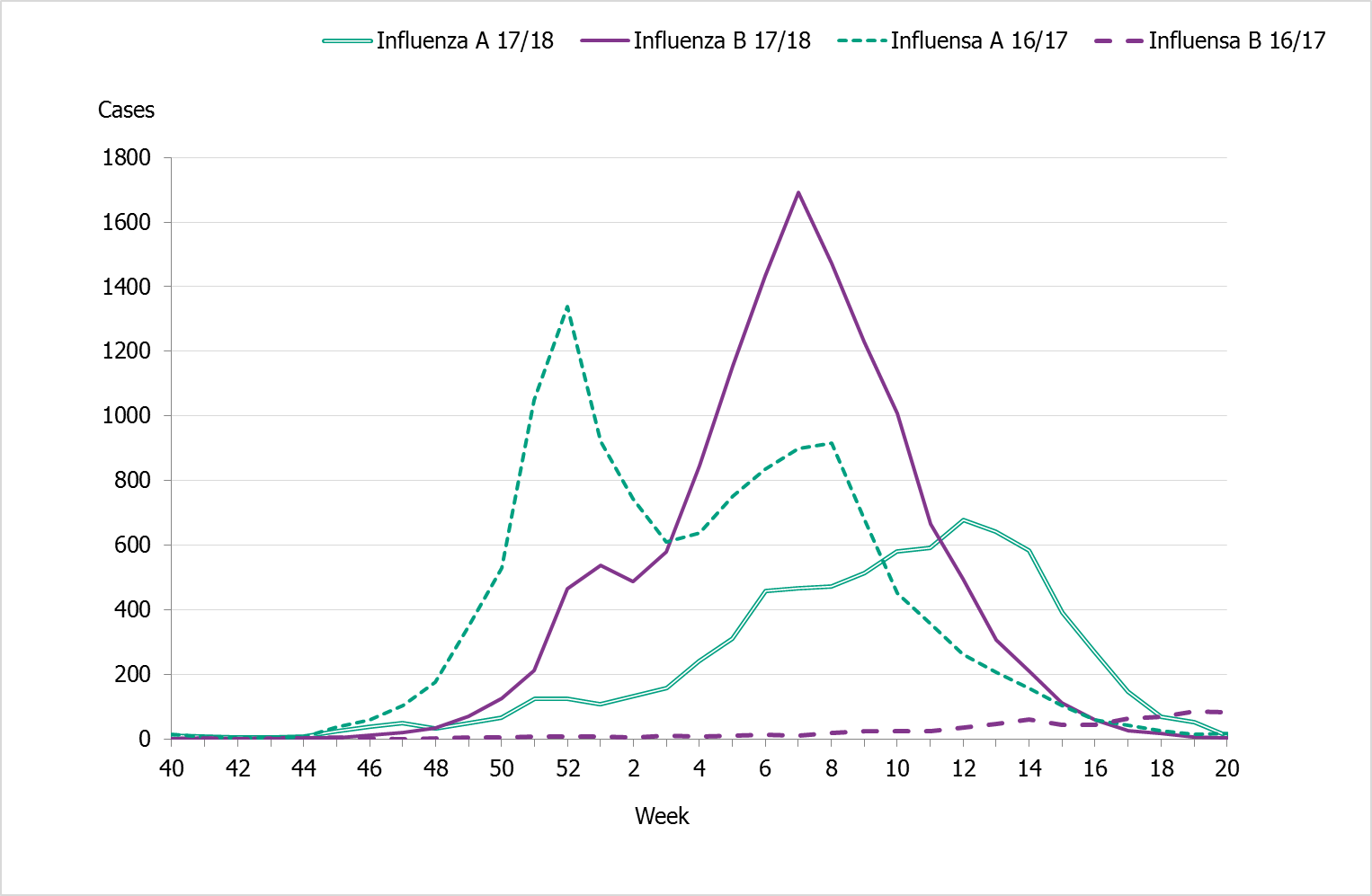
Figure 3. Total number of laboratory-confirmed cases of influenza (all types) per week and the dominating influenza type(s) per season, 2014–2018.
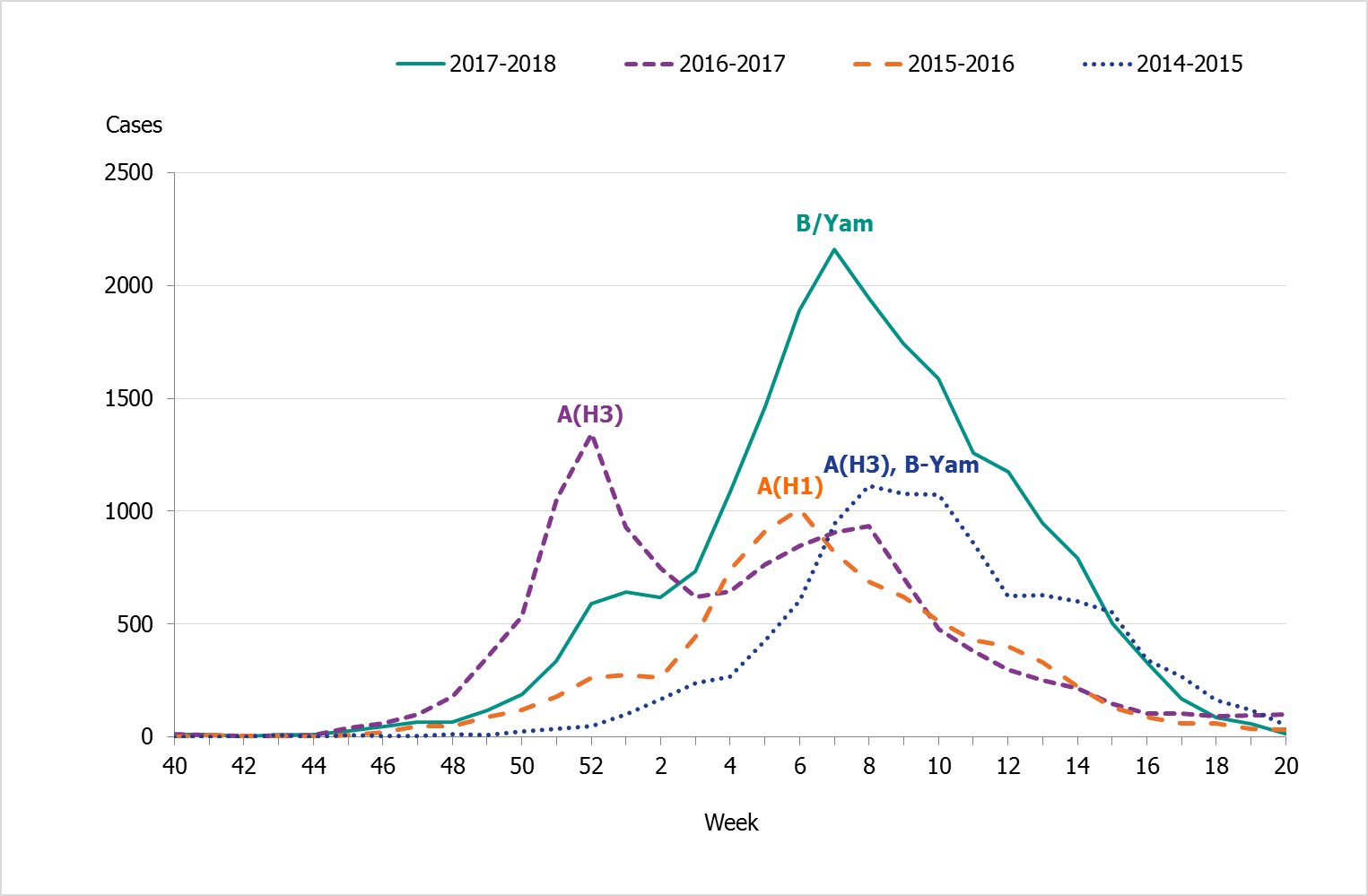
Figure 4. Percentage of samples testing positive for influenza, per week, 2014–2018.
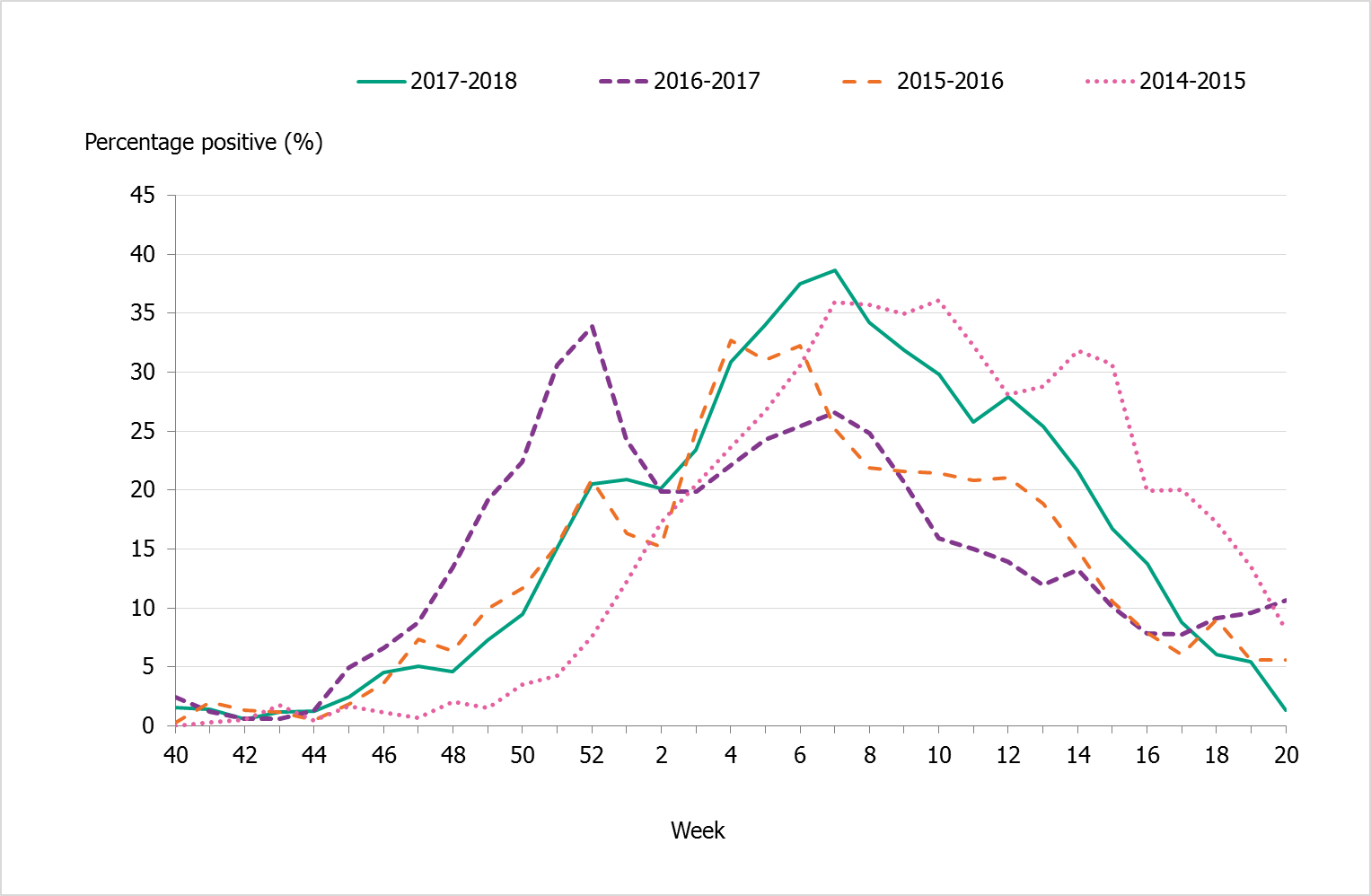
Figure 5. Number of laboratory-confirmed influenza cases stratified by level of care at sampling, 2015–2016 to 2017–2018.
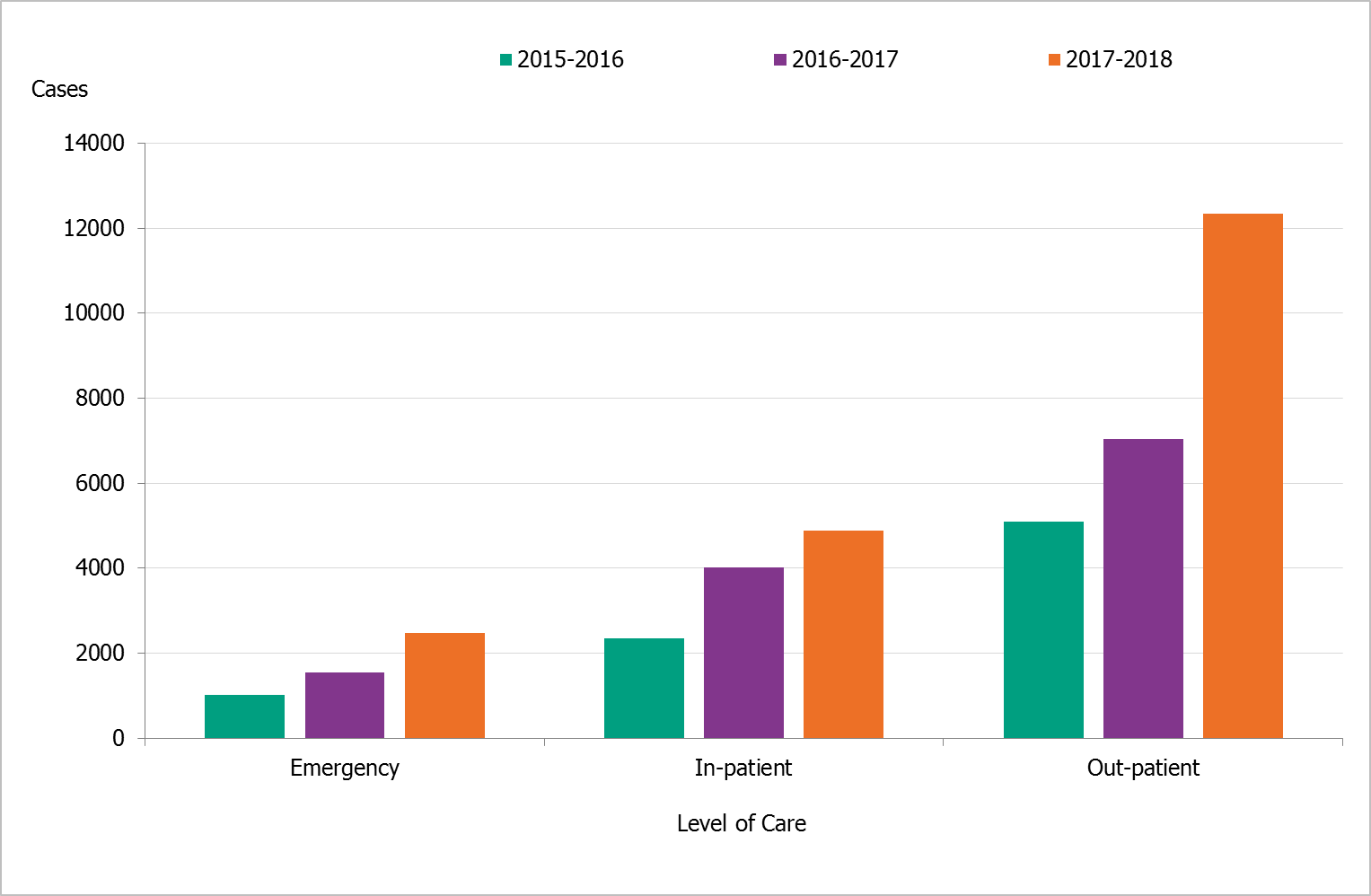
Level of care could not be determined for a small number of cases, which have been excluded.
Viral distribution
The 2017–2018 season was dominated by influenza B, with 13,280 laboratory-confirmed cases (64% of all cases). In total, 7,406 cases of influenza A were reported (36% of all cases). Samples of influenza B assigned to a lineage were almost exclusively B/Yamagata (99%). Of the subtyped influenza A-positive samples, 65% were A(H3N2) and 35% were A(H1N1)pdm09 (see also the Subtyping and lineage determination section). Table 3 summarises the laboratory reporting results over the last five seasons, including the number of analysed samples and the proportion of positive samples as well as the total samples positive by type, subtype, and lineage.
| 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | |
|---|---|---|---|---|---|
| Analysed samples | 22,330 | 42,668 | 48,135 | 68,241 | 88,837 |
| Proportion positive samples | 12% | 24% | 19% | 19% | 23% |
| Total positive for influenza A | 2,372 | 6,671 | 6,730 | 12,361 | 7,406 |
| A(H1N1)pdm09 * | 1,737 | 663 | 2,031 | 14 | 294 |
| A(H3N2) | 169 | 2,052 | 112 | 2,061 | 556 |
| A, not subtyped** | 466 | 3,956 | 4,587 | 10,286 | 6,556 |
| Total positive for influenza B | 213 | 3,718 | 2,409 | 708 | 13,280 |
| B/Victoria lineage*** | 2 | 2 | 65 | 11 | 0 |
| B/Yamagata lineage*** | 24 | 63 | 6 | 30 | 144 |
| B, not typed to any lineage | 187 | 3,653 | 2,338 | 667 | 13,136 |
* Not typed as N1, but classified as A(H1N1)pdm09 based on H1 typing.
** For the period 2013–2015, influenza A cases not subtyped but A(H1N1)pdm09 negative were considered to be influenza A(H3N2) cases. Data on subtype for the 2015–2016 and 2016–2017 seasons are from the Public Health Agency and the three regional laboratories that regularly perform subtyping.
*** All typing for lineage was performed at the Public Health Agency laboratory.
Age and sex distribution
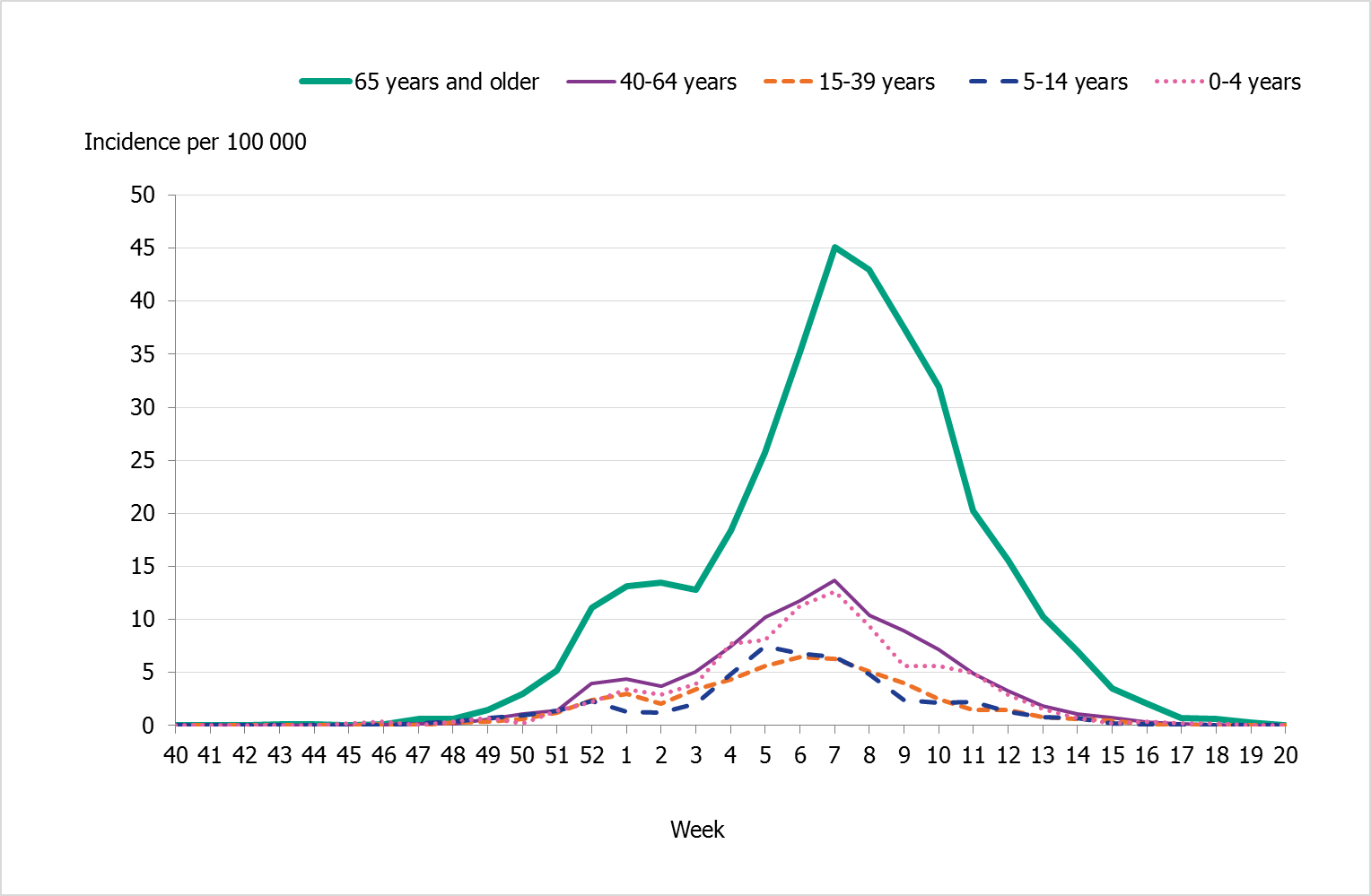
During the 2017–2018 season, most of the laboratory-confirmed influenza cases were in individuals aged 65 years and older, just like in previous seasons (Figure 6). Influenza B/Yamagata is previously known for affecting children and young adults when it circulates in the community. These younger individuals, however, usually recover at home and do not need to seek medical care for influenza. The age distribution of the laboratory-confirmed cases thus only reflects the burden in terms of individuals who have sought care for their influenza infection.
More than half (55%) of the laboratory-confirmed influenza B cases were among those aged 65 years and older, followed by individuals aged 40–64 years (24%) (Table 4). Those aged 65 years and over also had the highest incidence with 359 cases per 100,000 individuals, while those aged 40–64 years had an incidence of 103 cases per 100,000 individuals. Among the elderly, individuals aged 75–79 and 80-84 years had the greatest proportion of cases, and the age group 90–94 years had the highest incidence of all age groups for both influenza A and B (see Table 5).
In week 12, the dominance shifted from influenza B to influenza A. The age group 65 and older was also most affected by influenza A, with 58% of the laboratory-confirmed cases and an incidence of 211 cases per 100,000 individuals. The next highest incidence was seen among children aged 0–4 years, with an incidence of 78 cases per 100,000 individuals.
The median age for individuals with laboratory-confirmed influenza B was 68 years and for influenza A was 70 years (see Table 6). Significantly more women (52%) than men (48%) had laboratory-confirmed influenza (p = 0.0000).
Figure 6. Weekly incidence of influenza B per age group in Sweden, 2017–2018 season.
| 2012–2013 | 2014–2015 | 2017–2018 | ||||
|---|---|---|---|---|---|---|
| Age group | Incidence | Percentage | Incidence | Percentage | Incidence | Percentage |
| 0–4 | 26.0 | 5% | 16.4 | 3% | 87.0 | 4% |
| 5–14 | 32.6 | 12% | 14.6 | 4% | 50.8 | 5% |
| 15–39 | 19.2 | 21% | 19.4 | 16% | 53.1 | 13% |
| 40–64 | 31.4 | 34% | 40.3 | 34% | 102.6 | 24% |
| 65+ | 41.4 | 27% | 83.3 | 43% | 359.0 | 55% |
| Total | 29.2 | 100% | 37.8 | 100% | 131.3 | 100% |
| Note: Data do not include sentinel cases or cases where the age is unknown. | ||||||
| Age group | Population ‡ | Influenza A | Influenza B | ||
|---|---|---|---|---|---|
| Cases | Incidence | Cases | Incidence | ||
| 0–4 | 584,000 | 457 | 78.3 | 508 | 87.0 |
| 5–14 | 1,189,896 | 242 | 20.3 | 604 | 50.8 |
| 15–39 | 3,177,573 | 1020 | 32.1 | 1687 | 53.1 |
| 40–64 | 3,132,662 | 1416 | 45.2 | 3215 | 103 |
| 65–69 | 561,355 | 530 | 94.4 | 878 | 156 |
| 70–74 | 558,051 | 749 | 134 | 1316 | 236 |
| 75–79 | 378,503 | 816 | 216 | 1418 | 375 |
| 80–84 | 254,490 | 834 | 328 | 1446 | 568 |
| 85–89 | 166,201 | 779 | 469 | 1260 | 758 |
| 90–94 | 78,302 | 433 | 553 | 731 | 934 |
| ≥95 | 23,003 | 126 | 548 | 202 | 878 |
| Total | 10,104,036 | 7,402 | 73.3 | 13,265 | 131 |
*The table does not include sentinel cases or cases where age is unknown.
‡ Population on December 31, 2017. Source: Statistics Sweden, Statistikdatabasen.
| 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | |
|---|---|---|---|---|---|
| Influenza A | - | - | 48 | 74 | 70 |
| Influenza A(H1N1)pdm09 | 45 | 50 | - | - | - |
| Influenza A(H3N2)* | 58 | 72 | - | - | - |
| Seasonal influenza B** | 49 | 60 | 33 | 58 | 68 |
* For the period 2013–2015, all influenza A-positive samples that were negative for A(H1N1)pdm09 were classified as influenza A(H3N2). From 2015–2016 onward, median age is shown by type rather than subtype. ** The median age for influenza B-positive samples was calculated for both lineages because only a small portion of the samples were analysed for lineage.
Geographic distribution
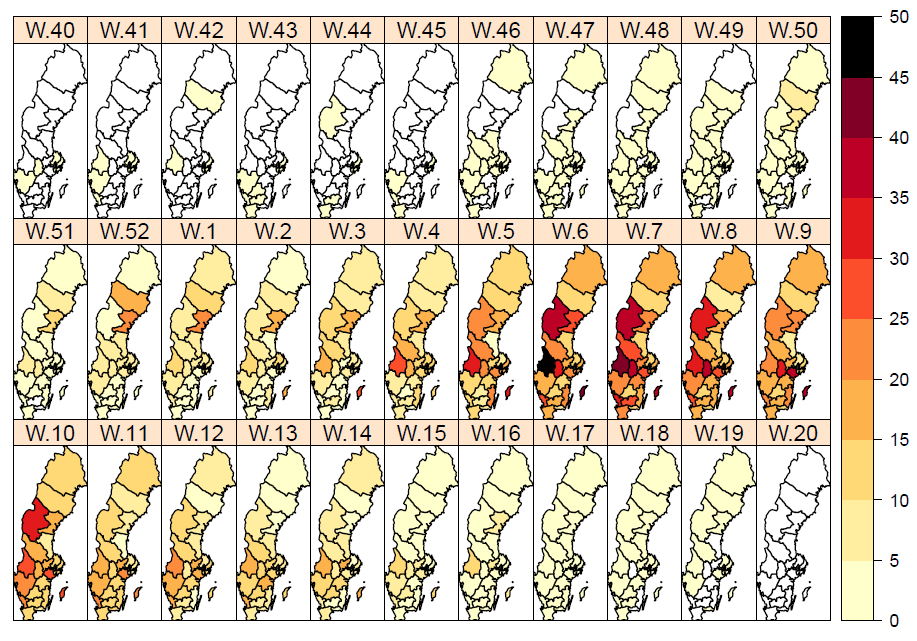
During the initial weeks of the epidemic, both the northern parts of Sweden (Norrland) and the middle parts of the country (Svealand) had higher incidence in comparison to the southern part (Götaland) of the country (Figure 7). During Christmas and New Year holidays (weeks 52–1), the incidence reached a first peak for the northern parts of the country (Norrland), and then a second peak was seen in week 6. The incidence in the middle (Svealand) and southern (Götaland) parts of the country peaked in week 7. The percentage of samples positive for influenza showed a similar geographic trend with peaks in weeks 52 and 6 for the northern part of the country (Norrland) and week 7 for the middle (Svealand) and southern (Götaland) parts of the country.
Altogether, the middle part of the country (Svealand) had the highest incidence per 100,000 individuals overall this season, with 223 cases, followed by the northern parts (Norrland) with 220 cases and the southern parts (Götaland) with 190 cases. The greatest numbers of cases was reported from the largest urban areas (Stockholm, Västra Götaland, and Skåne). However, in relation to population size, some smaller counties had a higher overall incidence. The number of laboratory-confirmed cases might be affected by health care seeking behaviour as well as differences in sampling in the various regions; thus, no direct conclusions can be drawn regarding actual influenza activity using the measured incidence.
Figure 7. Weekly incidence of laboratory-confirmed influenza per 100,000 population and county from week 40, 2017, to week 20, 2018. Note: The colour scale indicates the incidence. The scale has been changed from the previous season.
Antiviral sales
Every Monday, the Public Health Agency receives data from the Swedish eHealth Agency on the previous week's sales of the antivirals zanamivir and oseltamivir. Data include all sales categories, i.e. prescriptions and health care requisitions.
During the last two seasons, sales of antivirals have increased considerably and reached more than 20,000 packages in 2017–2018 (see Table 7). From 2016–2017 to 2017–2018, the number of prescriptions increased by 53 percent and the number of health care requisitions increased by 29 percent following the increase in laboratory-confirmed influenza cases. This is likely due in part to the age groups affected and reports of the mismatched influenza B strain in the vaccine. Antivirals were used for the treatment of ill patients in risk groups and in severely ill patients and as post-exposure prophylaxis in, for example, elder care facilities and hospital wards.
| 2013–2014 | 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | |
|---|---|---|---|---|---|
| Prescriptions | 1,445 | 3,610 | 3,720 | 4,806 | 7,371 |
| Health care requisitions | 1,819 | 5,389 | 4,930 | 10,262 | 13,195 |
| Total sales | 3,264 | 8,999 | 8,650 | 15,068 | 20,566 |
| Total influenza cases | 2,607 | 10,389 | 9,150 | 13,069 | 20,686 |
Influenza cases in intensive care
The Public Health Agency receives daily anonymised data on influenza patients in intensive care through a collaboration with the Swedish Intensive Care Registry (SIR). A special influenza module in the registry, known as SIRI, allows the treating physician at an intensive care unit to report underlying medical conditions, complications, antiviral treatment, vaccination status, influenza type, and other data for patients under treatment. In addition to the data available through SIRI, aggregated reports are also available at SIR's public web portal. Aggregated reports show all patients in intensive care who were diagnosed with influenza, either as primary or as secondary/other diagnosis, for patients whose intensive care has ended.
Data from SIRI
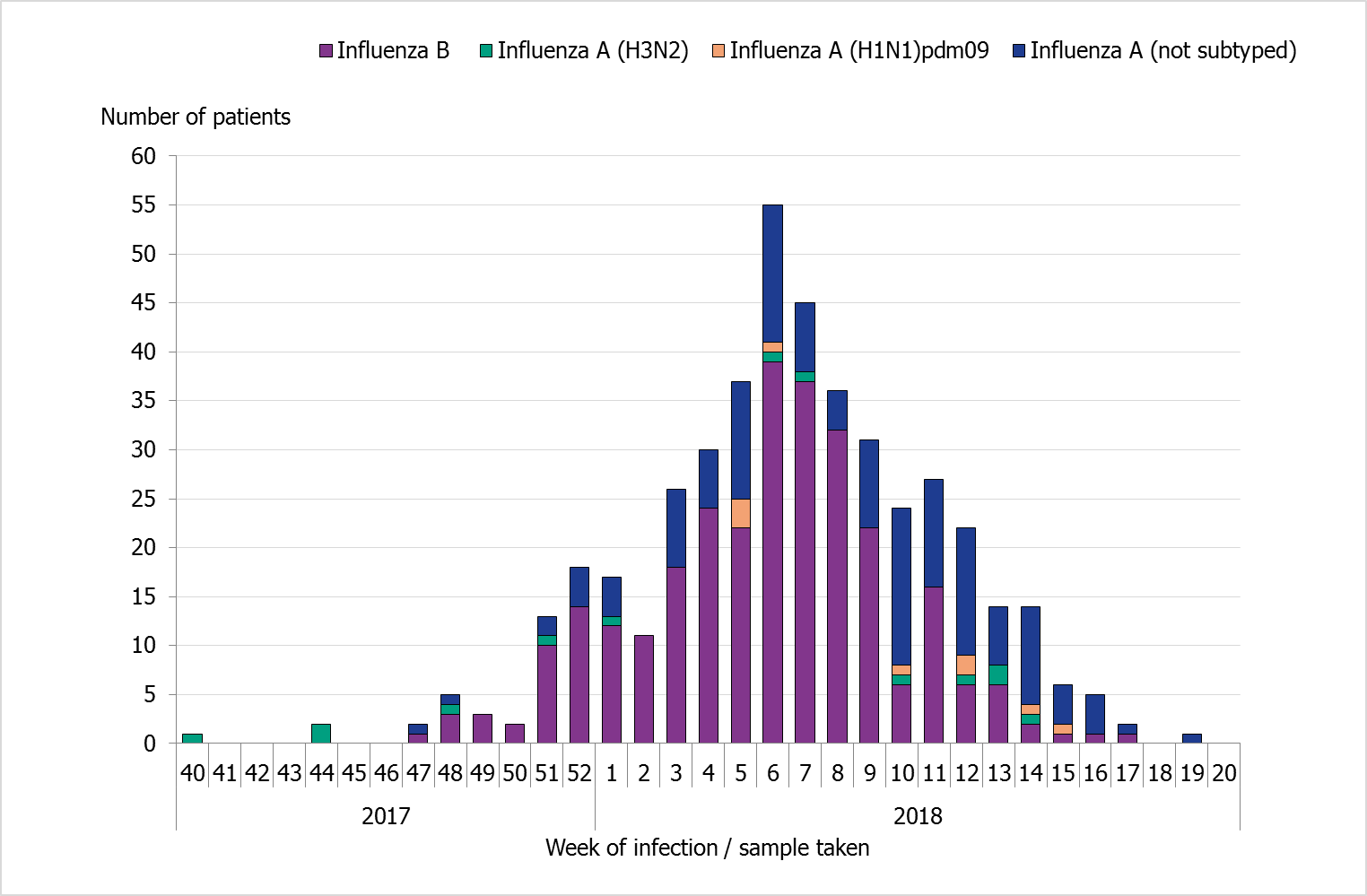
During the season, 447 patients with influenza were reported as having received intensive care. The majority of the cases had influenza B (288 cases), while 159 cases had influenza A (Table 8 and Figure 8). Of patients with reported influenza subtyping results, 9 patients had influenza A(H1N1)pdm09 and 13 patients had influenza A(H3N2). Most of the patients were admitted to intensive care during weeks 5–8, which corresponds to the weeks in which the most cases of laboratory-confirmed influenza were reported.
The age distribution of patients in intensive care under the age of 65 was similar to the season in 2015–2016, which was dominated by influenza A(H1N1)pdm09. Individuals aged 65 years and older were the most affected age group in 2017–2018 (234 patients), followed by the age group 40–64 years (144 patients). The median age for patients with influenza B was 67 years, while it was 64 years for patients with influenza A. There was no significant difference in sex distribution.
Of all reported cases, 332 patients (74%) were in a risk group for severe influenza illness, either due to age (65 years and older) or due to one or more medical risk factors. Among patients under the age of 65 years, more than half (116 patients, 54%) did not have a medical risk factor for severe influenza, which was similar to the previous season. However, the number of cases of younger individuals was larger in 2017–2018 (116 patients) compared with 2016–2017 (41 patients). Chronic heart-lung disease (n = 166), immunosuppression (n = 54), and chronic liver/kidney disease (n = 43) were the most commonly reported risk factors in the 2017–2018 season. One of the patients with influenza A was pregnant.
Altogether, 332 patients were recommended seasonal influenza vaccination due to either age or medical risk factor. Vaccination status was known for 190 patients, of which 44 (23%) were vaccinated. The majority of all vaccinated patients with influenza had influenza B (32 patients), while some had influenza A (12 patients). The protection against influenza B was lower this season because the dominating influenza B lineage B/Yamagata was not included in the trivalent seasonal vaccine, which was by far the most common type of vaccine in Sweden. The age for vaccinated patients ranged from 50 to 94 years, with a median age of 73 years. Vaccine effectiveness decreases with age, and the majority of the vaccinated patients were aged 65 years and older (38 of 44 patients). Vaccine effectiveness might vary at an individual level depending on factors such as age, immunity, and time from vaccination to time of infection. Moreover, vaccine effectiveness also depends on the level of matching between circulating and vaccine strains. Thus, influenza infection is seen among vaccinated individuals each season.
Information regarding primary diagnosis in intensive care was reported for 399 patients. Influenza with pneumonia (n = 62), septic shock (n = 45), and respiratory insufficiency (n=36) were the most common primary diagnoses for intensive care.
Of the patients requiring intensive care, 114 individuals died. The majority (90%) of the deceased had a medical risk factor or were aged 65 years or older and therefore were at increased risk of severe influenza infection. Among patients aged 40–64 years who died, 18 out of 25 had a medical risk factor for severe influenza infection. Two out of six patients under 40 years of age who died had a medical risk factor. The median age of all patients who died was 72 years.
Figure 8. Number of patients with influenza in intensive care by influenza type and number of laboratory-confirmed cases, 2017–2018 season.
| 2012–2013(n = 22) | 2013–2014(n = 24) | 2014–2015(n = 31) | 2015–2016(n = 53) | 2016–2017(n = 49) | 2017–2018(n = 53) | |
|---|---|---|---|---|---|---|
| Cases (median age) | ||||||
| Influenza A (not subtyped) | * | * | * | 157 (56) | 196 (73) | 136 (64) |
| Influenza A(H1N1)pdm09 | 67 (56) | 54 (59) | 18 (63) | 156 (58) | 3 (82) | 9 (59) |
| Influenza A(H3N2) | 36 (64) | 7 (63) | 103 (70) | 4 (56) | 50 (72) | 14 (72) |
| Influenza B | 34 (58.5) | 1 (±) | 55 (54) | 50 (52) | 9 (66) | 288 (67) |
| Total | 137 | 62 | 176 | 367 | 258 | 447 |
| * From season 2012–2013 to 2014–2015, all samples of influenza A were subtyped for influenza A(H1N1)pdm09. Thus, it is assumed that all other influenza A cases were A(H3N2). As of the 2015–2016 season, subtyping is no longer required. ± No median age shown for reasons of patient privacy. | ||||||
Five seasons of SIRI surveillance
Age distribution
The age distribution of patients in intensive care has varied during the past five seasons (Figure 9), and the number of reporting intensive care units has increased. The number of patients aged 65 years and older has increased each season. During 2017–2018 as well as season 2015–2016, there were many cases aged 40–64 years, as well as more cases reported among children and young adults than the other seasons. Although the number of cases among ages 40–64 years has varied over the years, the proportion of patients in a risk group in this age group has been stable (51–54%) during the last three seasons. In contrast to previous seasons, the proportion of patients in risk group among patients under the age of 40 was lower in season 2017–2018, and 72 percent of patients under 40 years did not have a risk factor for severe influenza infection.
Figure 9. Age distribution of patients in intensive care with influenza during five seasons, 2013–2018.
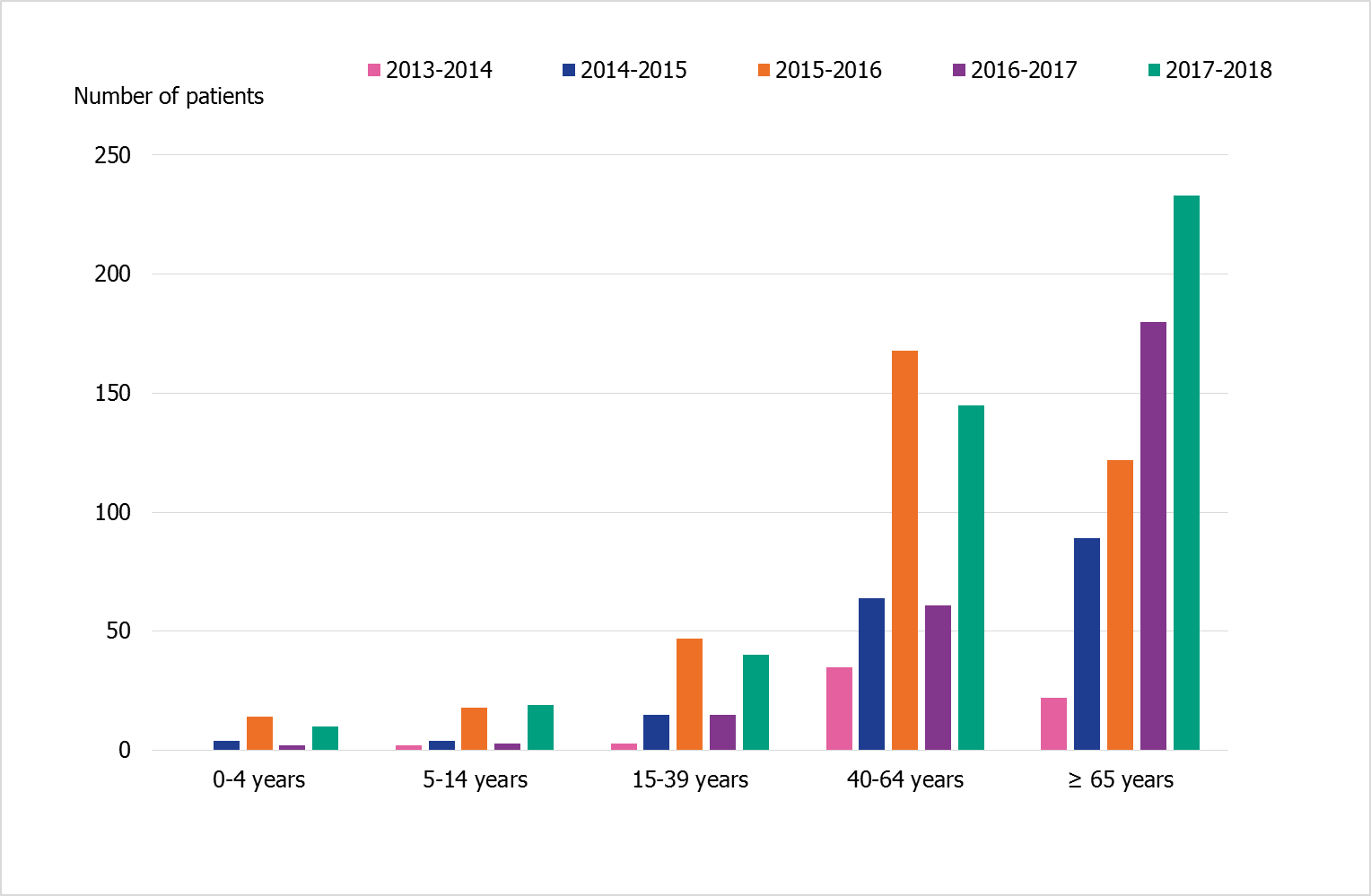
More intensive care units started reporting in the later part of the period shown. Therefore, the number of patients in intensive care with influenza is likely underestimated during the first two seasons shown. The distribution of patients among influenza type and subtypes for each season is shown in table 8.
ECMO-treatment
During 2017–2018, 15 patients received extracorporeal membrane oxygenation (ECMO), which is a slight increase in comparison to the intense 2015–2016 season, during which 12 patients were treated with ECMO. In particular, there were more patients under the age of 40 (9 patients) receiving ECMO treatment in comparison to 2015–2016 (1 patient). Most (approx. 80 percent) of the patients receiving ECMO did not have a medical risk factor for severe influenza infection. The number of cases aged 40–64 years (5 patients) was lower than 2015–2016 (10 patients). These five patients lacked any reported medical risk factors for severe influenza, which is in contrast to previous seasons, where 43–50 percent had at least one medical risk factor.
Mortality
The age distribution of patients in intensive care is reflected among those intensive care patients who have died (Figure 10). The majority of the patients who have died during this and the previous three seasons have been aged 65 years and older. Deaths have also occurred each season among patients aged 40–64 years, and of those 63–73 percent have had at least one medical risk factor for severe influenza. A few deaths among teenagers and young adults with at least one medical risk factor for severe influenza have been reported in previous seasons. In contrast to previous seasons, four patients under the age of 40 without any reported medical risk factor died during 2017–2018. Two had influenza A and two had influenza B.
Figure 10. Age distribution of deaths among patients in intensive care with influenza during the last five seasons, 2013–2018.
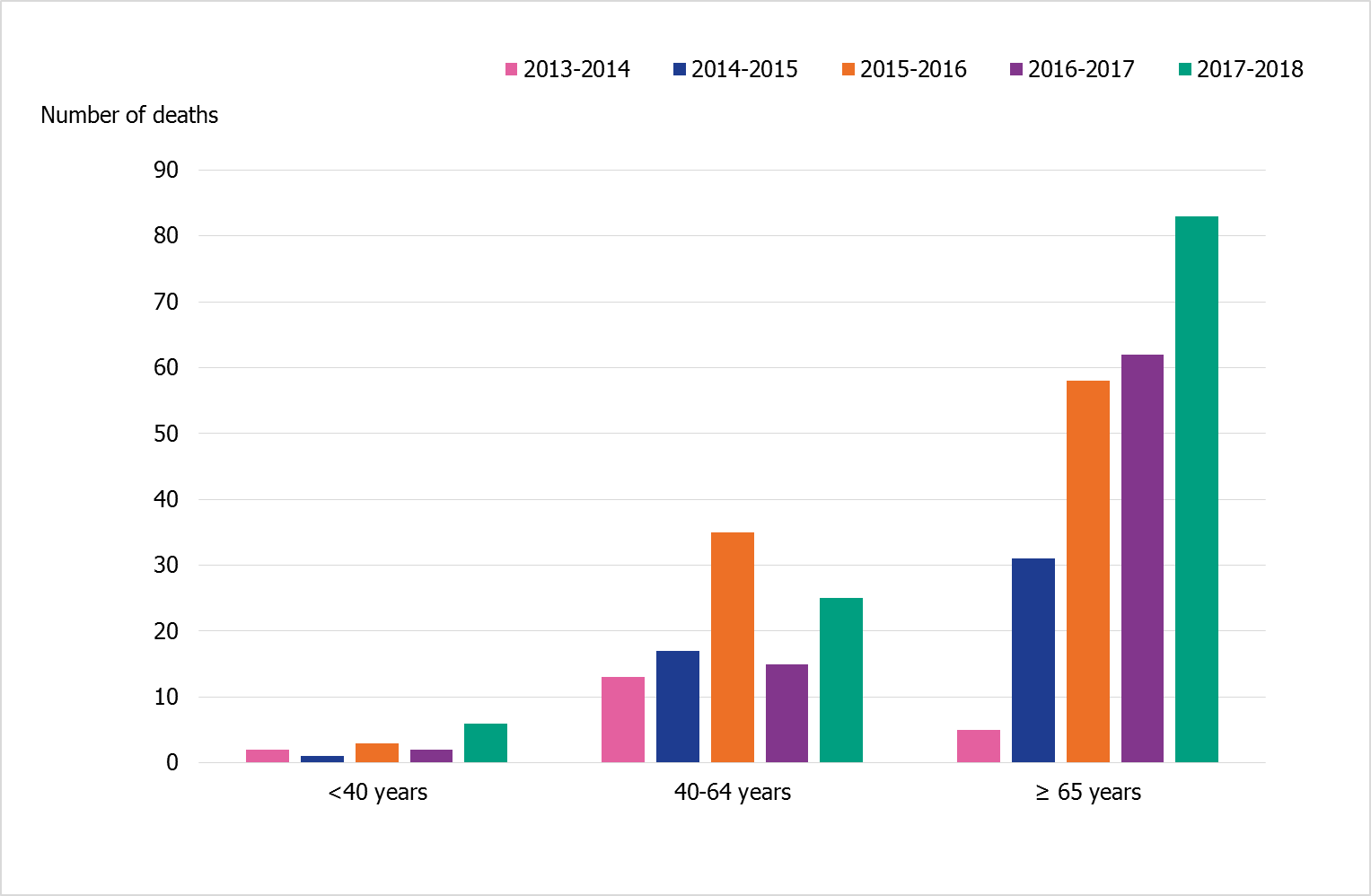
See note under previous figure.
Evaluation of SIRI
Most intensive care units (89 units) are connected to SIR and as such are able to report to SIRI, but this reporting is voluntary. The number of units registered for reporting with SIR has remained relatively constant during the period 2012–2018. A minor evaluation has been done with regards to coverage of SIRI surveillance. Data are reported from both SIRI and SIR with some delay, thus this evaluation is preliminary.
The number of units that have reported to SIRI for at least one season has increased from 19 to 60 units since SIRI surveillance started in 2012–2013. An increase in the number of units reporting to SIRI occurred in 2015–2016, and since then the number of reporting units has been stable at 49–53 units. During the 2017–2018 season, 53 units reported to SIRI. The increase in the number of reporting units makes it difficult to compare the crude number of patients in intensive care over time.
An evaluation restricted to the past three seasons shows that there were more patients with influenza who required intensive care in 2017–2018 compared to the two previous seasons. When comparing the number of cases reported from only those units that have reported in all three previous seasons (2015–2016, 2016–2017, and 2017–2018) (n = 40), the number of patients was 314, 230, and 377 per season, respectively. Many patients received intensive care during both the last season and 2015–2016. The units reporting in both 2016–2017 and 2017–2018 (n = 44) reported 247 and 413 cases per season, respectively.
Data from SIR
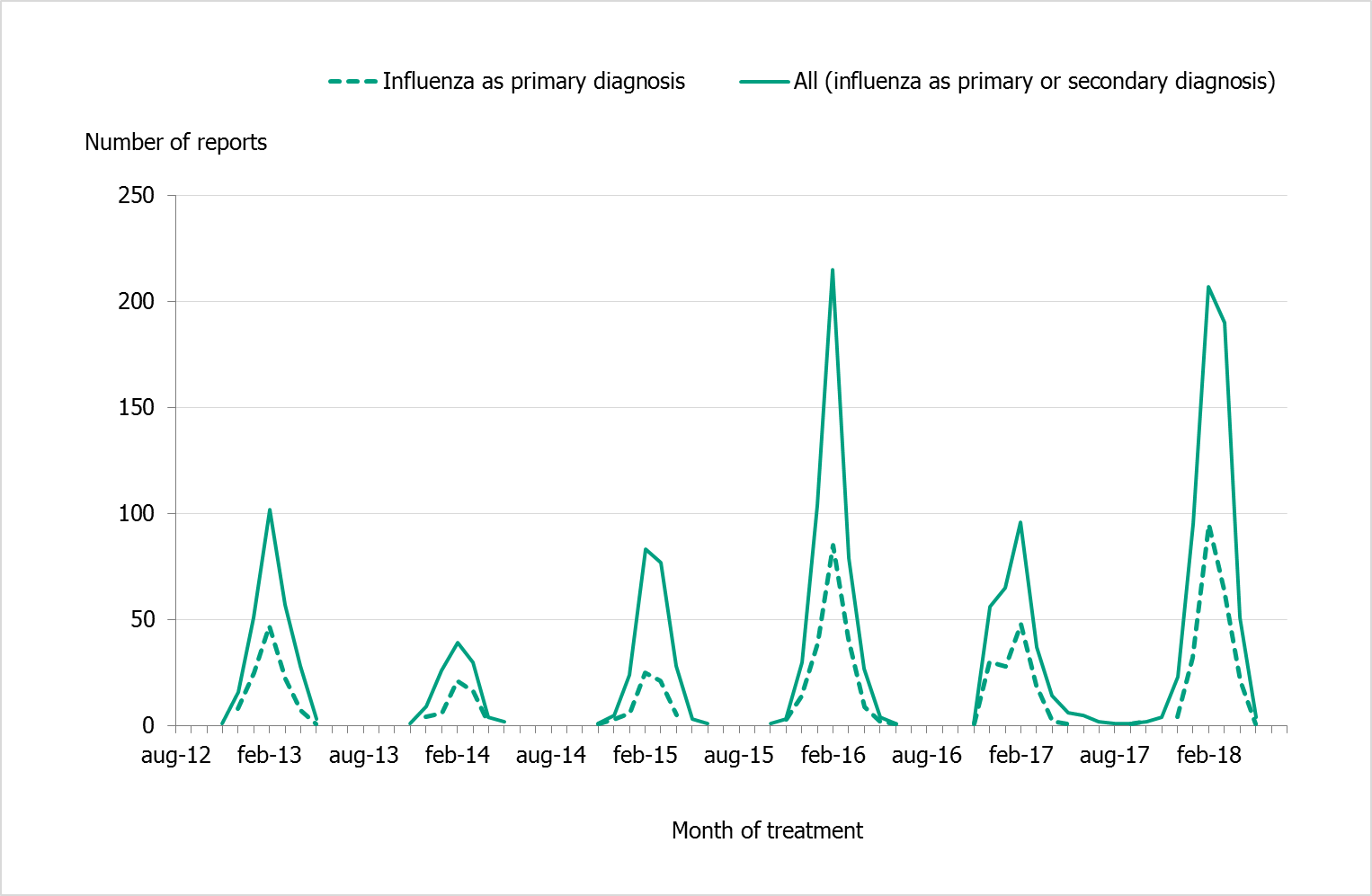
In addition to the data available through SIRI, aggregated reports are also available at SIR's public web portal. The aggregated reports show all patients in intensive care who were diagnosed with influenza, either as the primary or as the secondary/other diagnosis, and whose intensive care has ended. Data from SIR also show that the number of patients with influenza receiving intense care increased during the 2017–2018 season in comparison to previous seasons (Figure 11).
A total of 447 patients were reported to SIRI during 2017–2018, and of these 404 had been discharged or had died (as of July 9, 2018). Comparing the data from SIRI and SIR can give an indication of the coverage of SIRI surveillance. During the last season, 72 percent of the cases reported to SIR were also reported to SIRI. The coverage of SIRI has varied between 70 and 90 percent since 2014–2015. Table 9 shows the crude number of intensive care patients with laboratory-confirmed influenza reported to SIR and SIRI, respectively, over the past six seasons.
Figure 11. Number of patients with laboratory-confirmed influenza who received intensive care per season, 2012–2018.
| Total in SIR | Total in SIRI | Proportion of cases reported to SIRI | |
|---|---|---|---|
| 2012–2013 | 258 | 135 | 52% |
| 2013–2014 | 111 | 54 | 49% |
| 2014–2015 | 223 | 176 | 79% |
| 2015–2016 | 466 | 367 | 79% |
| 2016–2017 | 283 | 258 | 91% |
| 2017–2018 | 578 | 404* | 70% |
| * Although a total of 447 patients were reported through SIRI, 43 of these patients had not ended their care (as of July 9, 2018) and are thus excluded from this comparison because SIR data only include patients whose intensive care has ended. | |||
Influenza-related mortality
Influenza-related mortality is often noted during influenza seasons, but it varies depending on the circulating strain and the intensity of the season. The Public Health Agency uses different systems to measure influenza-related mortality.
Data on laboratory-confirmed influenza patients are intermittently linked to Swedish Tax Agency data on death to identify deceased individuals and to retrieve their dates of death. If 30 days or fewer have elapsed since the influenza diagnosis, the death is considered to be influenza-related. This measurement is imprecise because the death might have been caused by something else. Importantly, this measure excludes anyone who might have died from influenza without getting a laboratory-confirmed diagnosis, meaning that there is most likely a large number of unrecorded deaths from influenza.
To identify otherwise unrecorded deaths from influenza, two models are used to identify crude and influenza-related excess mortality during the influenza season using the aggregate number of deaths. The EuroMoMo model estimates the crude excess mortality for the whole country by age group and regionally. The FluMoMo model estimates excess mortality due to either influenza activity or extreme temperatures, both nationally and by age group.
Deaths 30 days after influenza diagnosis
In total, 1,021 of 20,857 persons who received a laboratory-confirmed influenza diagnosis during the 2017–2018 season died within 30 days of diagnosis. Of these, 339 had influenza A and 682 had influenza B. Most samples of influenza A and B were not analysed for subtype or lineage, respectively. Most (77 percent) of those who died did so within 15 days of diagnosis.
The vast majority of deaths within 30 days occurred among people aged 65 years and older (93 percent), while 6 percent occurred among adults aged 40–64 years and less than 1 percent occurred in people under the age of 40 years. Patients who died ranged in age from 1 to 103 years, with a median age of 84 years of age. Patients who had not died within 30 days of diagnosis had a median age of 69 years.
Overall, 8.2 percent of those aged 65 years and older who received a laboratory-confirmed influenza A diagnosis died within 30 days. Table 10 shows that the proportion of deaths within 30 days increased with increasing age and varied from 0.2 percent for persons aged under 40 years to 21% for people aged 95 years and older. The analyses have not been adjusted for expected mortality in each age group.
| <40 years> | 40–64 years | 65–69 years | 70–74 years | 75–79 years | 80–84 years | 85–89 years | 90–94 years | ≥95 years | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cases | 4,598 | 4,724 | 1,427 | 2,064 | 2,231 | 2,283 | 2,038 | 1,164 | 328 | 20,857 |
| Cases/100,000 | 93 | 151 | 254 | 370 | 589 | 897 | 1,226 | 1,487 | 1426 | 6,493 |
| Total Deaths | 7 | 65 | 48 | 97 | 136 | 185 | 209 | 204 | 70 | 1,021 |
| Deaths/100,000 | 0.1 | 2 | 9 | 17 | 36 | 73 | 126 | 261 | 304 | 827 |
| Deaths among cases (%) | 0.2% | 1% | 3% | 5% | 6% | 8% | 10% | 18% | 21% | 4.9% |
| This analysis includes all laboratory-confirmed influenza cases from week 40, 2017, to week 20, 2018. It excludes 296 patients whose personal identification number was not included in the case report, meaning their status at 30 days could not be ascertained. | ||||||||||
Excess mortality
The FluMoMo model measured elevated influenza-related mortality for the age group 65 years and older for 13 weeks (weeks 3–15, 2018), see Table 11 and Figure 12. Influenza-related excess mortality is most frequently noted in this age group. The excess mortality this season was on the same order of magnitude as in the 2016–2017 season when influenza A(H3N2) significantly affected those aged 65 years and older. The period of elevated excess mortality this season included both the intense influenza B epidemic and the second, smaller wave of influenza A. It is unusual that a season dominated by influenza B causes such high excess mortality because influenza B does not usually cause major epidemics, but this confirms that the elderly are the most vulnerable age group in terms of the risk of dying from influenza.
At the European level, excess mortality has been reported among those aged 65 years and older as well as, to a lesser extent, in the age group 15–64 years (see EuroMoMo project (9)).
| Season | Week | Age Group |
|---|---|---|
| 2011–2012 | Week 7, 2012 | 15–64 years |
| Weeks 7–14, 2012 | ≥65 years | |
| 2012–2013 | Weeks 5–9 and 11, 2013 | ≥65 years |
| 2014–2015 | Weeks 5–16, 2015 | ≥65 years |
| 2015–2016 | Week 4–6, 2016 | 15–64 years |
| 2016–2017 | Week 52, 2016, to week 11, 2017 | ≥65 years |
| 2017–2018 | Weeks 3–15, 2018 | ≥65 years |
| Notes: No significant excess mortality was seen in any age group or in the total population during the 2010–2011, 2013–2014, or 2015-2016 seasons. Each week, the FluMoMo model is updated to include the past 290 weeks (approximately 5.5 years). Estimates in this report are based on the most recent modelling for 2013–2014 onward. Estimates of excess mortality for the period from 2009 to 2012–2013 are based on a model including all data from 2009 to 2018. Since the previous annual report, the FluMoMo model has been adjusted, which has meant the baseline for expected deaths has been modified, among other things. Weeks shown in grey correspond to weeks where previous models indicated significant excess mortality occurred, but where the mortality levels are within the bounds of expected mortality in the new model. The updated model now shows significantly elevated influenza-related excess mortality among those 65 years or older in the 2012–2013 season – a level of excess mortality that was previously not considered significant, while excess mortality in the age group 15–64 years in 2012 and 2016 is no longer significantly elevated. | ||
Figure 12. Number of deaths per week (grey), influenza-related excess mortality (red), and temperature-related excess mortality (green) in each age group and in total, Sweden, 2013–2018 (week 20).
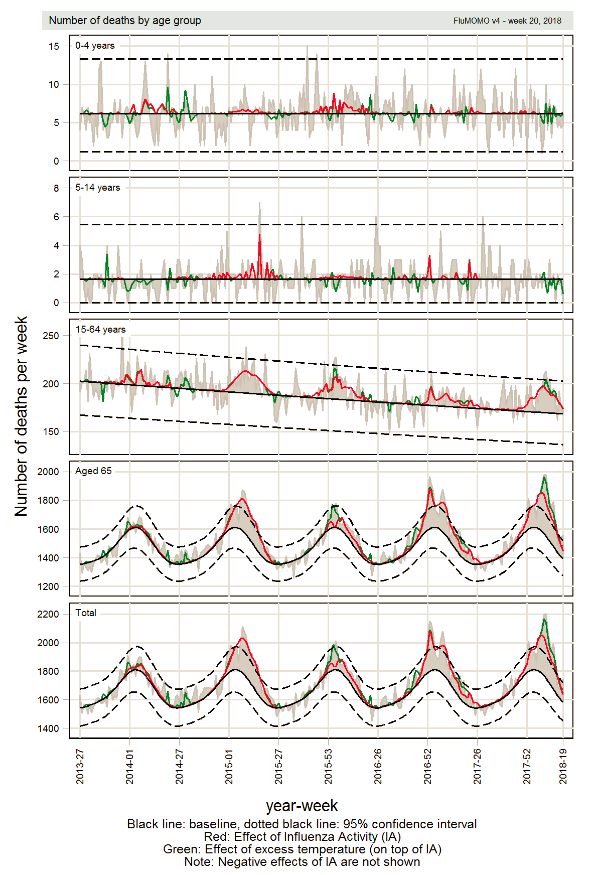
Note: The expected numbers of deaths are shown in black, the actual numbers of deaths in grey, influenza-related excess mortality in red, and temperature-related excess mortality in green, as estimated by the FluMoMo model. Some variation in the number of deaths is expected week to week, which is illustrated with the dashed lines marking two standard deviations around the estimated number of expected deaths per week. Estimated excess mortality exceeding these boundaries is considered a significantly elevated excess mortality.
Sentinel sampling
Virological analysis of sentinel samples from sentinel general practitioners, infectious disease clinics, and paediatric clinics contributes to national and international surveillance of circulating influenza viruses. In order to estimate what proportion of the patients seeking care for ILI actually has influenza, the different clinics are encouraged to collect nasal samples from patients with ILI. Patient characteristics, including age, sex, risk factors, syndrome (ILI vs. acute respiratory illness (ARI)), and vaccination status, are analysed with respect to the types of influenza that are circulating. The Public Health Agency carries out laboratory analyses for influenza free of charge for these samples. Representative positive samples are also used to characterise the circulating strains of influenza.
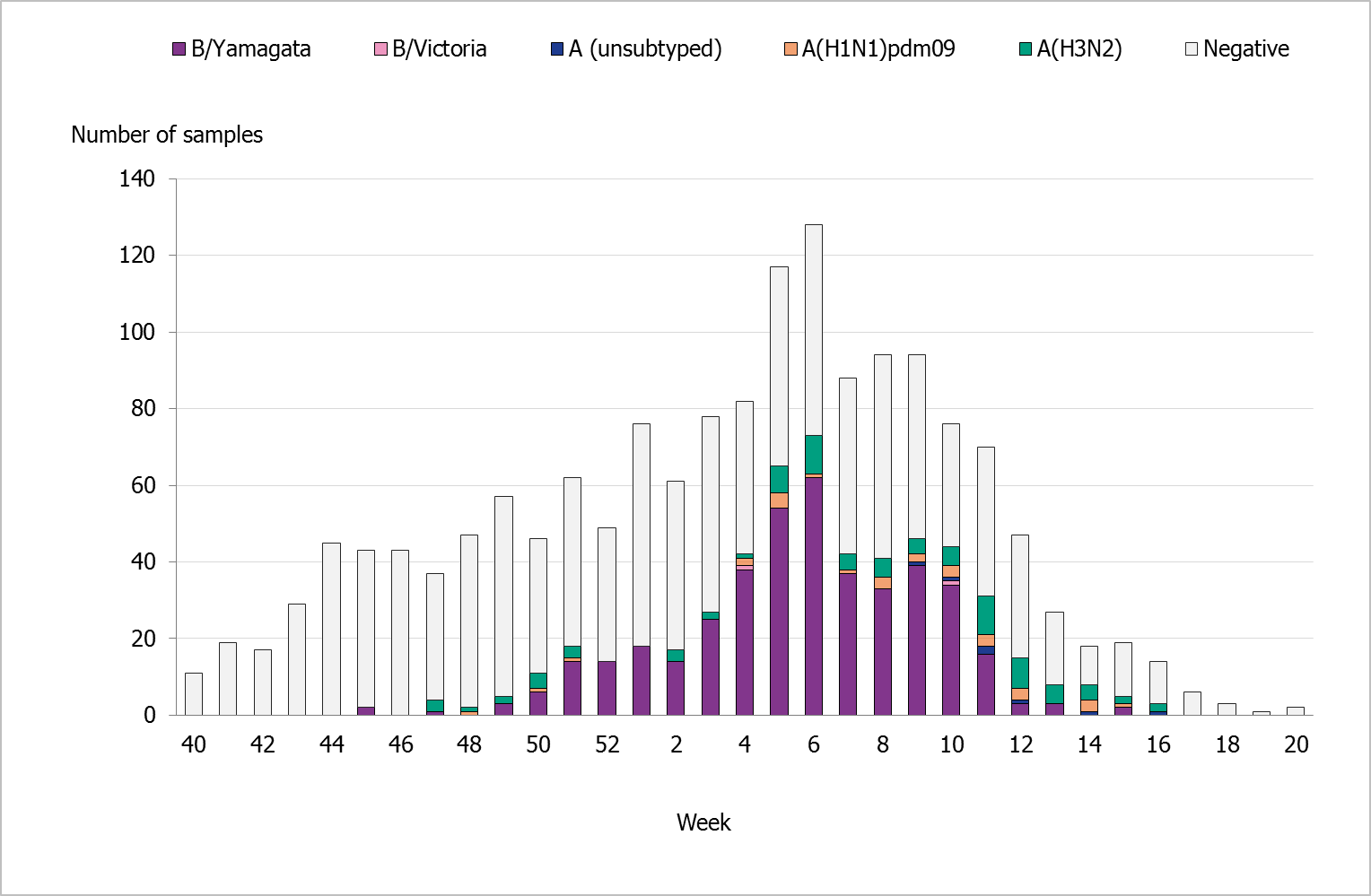
During the 2017–2018 season, 1,616 sentinel samples were submitted from 78 participants, including 71 general practitioners and 7 paediatric or infectious disease clinics. Sixty-eight percent of the samples were collected by general practitioners. In total, 541 samples (33%) tested positive for influenza. Figure 13 shows the distribution of samples taken and positive samples by subtype / lineage.
Of the positive samples, 124 (23%) were positive for influenza A, and 417 (77%) were positive for influenza B. Of the subtyped influenza A-positives samples, 87 (70%) were influenza A(H3N2) and 28 (23%) were influenza A(H1N1)pdm09. Nine influenza A samples (7%) could not be subtyped due to low virus concentration. In total, 417 samples were positive for influenza B. Of these, 415 samples belonged to the B/Yamagata/16/88 lineage, one belonged to the B/Victoria/2/87 lineage, and one sample was positive for both lineages. The last sample came from an unvaccinated adult belonging to a medical risk group.
Figure 13. Number of sentinel samples submitted each week and the number of samples by subtype/lineage, 2017–2018.
Clinical features
Of the 1,616 patients sampled through the sentinel system with known symptoms, 90 percent had ILI (Table 12) and 10 percent had ARI. In total, 59 percent of the samples came from women. Twenty percent of the samples were collected from patients belonging to a risk group due to age and/or medical condition. Of these, 24 percent were aged 65 years or older. The most common reported medical risk factors were heart disease (n = 35), lung disease (n = 20), and diabetes (n = 25).
| Season 2015–2016 | Season 2016–2017 | Season 2017–2018 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Medianage | ILI % | Number | Medianage | ILI% | Number | Medianage | ILI% | |
| Total analysed | 1,341 | 43.5 | 82% | 1,120 | 36 | 85% | 1,616 | 42.5 | 85% |
| Negative | 972 | 887 | 1075 | ||||||
| Proportion positive | 28% | 21% | 33% | ||||||
| Influenza A | 271 | 227 | 124 | ||||||
| A(H1N1)pdm09 | 259 | 28.5 | 86% | 2 | 27 | 100% | 28 | 35 | 71% |
| A(H3N2) | 11 | 40 | 100% | 213 | 34 | 90% | 87 | 43 | 85% |
| A, not subtyped | 1 | - | 100% | 12 | 35 | 93% | 9 | 50 | 89% |
| Influenza B | 97 | 6 | 417 | ||||||
| B/Victoria | 89 | 18 | 84% | 2 | 4 | 100% | 1 | - | 100% |
| B/Yamagata | 8 | 47 | 75% | 3 | 44 | 100% | 415 | 41 | 89% |
| B, both lineages | 0 | 1 | - | 0,0% | 1 | - | 100% | ||
Influenza infection among vaccinated patients
Vaccination status was reported for 1,587 (98%) of the 1,616 patients sampled during the season. Of these, 201 (13%) were vaccinated. Among the patients belonging to a risk group, 42% were vaccinated. Influenza was detected among 63 vaccinated patients, of which 16 had influenza A and 47 had influenza B.
Among the 16 vaccinated patients with influenza A, influenza A(H3N2) was detected in 13 patients and A(H1N1)pdm09 in 2 patients, see Table 13. One patient had onset of influenza A disease less than two weeks after influenza vaccination, but because full immunity is not obtained in that time period, the patient was excluded from the analysis. Twelve of the vaccinated patients with influenza A were aged 65 years and older, and the median age for all vaccinated influenza A cases was 71 years. Of the five patients younger than 65 years of age, three patients belonged to a medical risk group.
All of the 47 influenza B-positive samples from vaccinated patients were B/Yamagata lineage. The median age for these cases was 68 years. In total, 25 of the patients belonged to a risk group due to age or medical condition. The B component in the seasonal trivalent vaccine was B/Victoria, which explains the higher number of vaccinated patients with B/Yamagata.
The Public Health Agency of Sweden participates in I-MOVE (European Influenza - Monitoring Vaccine Effectiveness) with the sentinel sampling data. The interim I-MOVE results indicate that the 2017–2018 influenza vaccine effectiveness in all age groups was 25–52 percent against any influenza, 55–68 percent against influenza A(H1N1)pdm09, ≤7 percent against influenza A(H3N2), and 36–54 percent against influenza B (10). The moderate vaccine effectiveness against circulating B/Yamagata indicates important cross-lineage protection from B/Victoria in the trivalent vaccine used in Sweden during this season.
| Season 2015–2016 | Season 2016–2017 | Season 2017–2018 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Medianage | ILI % | Number | Medianage | ILI% | Number | Medianage | ILI% | |
| Total analysed | 1,341 | 43.5 | 82% | 1,120 | 36 | 85% | 1,616 | 42.5 | 85% |
| Negative | 972 | 887 | 1075 | ||||||
| Proportion positive | 28% | 21% | 33% | ||||||
| Influenza A | 271 | 227 | 124 | ||||||
| A(H1N1)pdm09 | 259 | 28.5 | 86% | 2 | 27 | 100% | 28 | 35 | 71% |
| A(H3N2) | 11 | 40 | 100% | 213 | 34 | 90% | 87 | 43 | 85% |
| A, not subtyped | 1 | - | 100% | 12 | 35 | 93% | 9 | 50 | 89% |
| Influenza B | 97 | 6 | 417 | ||||||
| B/Victoria | 89 | 18 | 84% | 2 | 4 | 100% | 1 | - | 100% |
| B/Yamagata | 8 | 47 | 75% | 3 | 44 | 100% | 415 | 41 | 89% |
| B, both lineages | 0 | 1 | - | 0,0% | 1 | - | 100% | ||
* Vaccinated less than 14 days before symptom onset and excluded from the analysis.** B/Yamagata was not included in the trivalent inactivated influenza vaccine.
Subtyping and lineage determination
All diagnostic laboratories perform influenza typing using molecular assays for influenza A and B. During the 2017–2018 season, subtyping was performed at three regional laboratories (Lund, Göteborg, and Uppsala). The Public Health Agency performs subtyping and lineage typing by real-time PCR for all samples sent in from the diagnostic laboratories and on all positive samples from sentinel surveillance.
In total, 850 influenza A-positive samples were subtyped during the season, of which 556 (65%) were A(H3N2) and 294 (35%) were A(H1N1)pdm09.
The lineage was determined for 144 influenza B-positive samples. All belonged to the B/Yamagata lineage. Subtyping results for sentinel reporting are presented in Table 14.
| 2014–2015 | 2015–2016 | 2016–2017 | 2017–2018 | |||||
|---|---|---|---|---|---|---|---|---|
| Influenza type | Sentinel | Lab | Sentinel | Lab | Sentinel | Lab | Sentinel | Lab |
| A(H1N1)pdm09 | 9% | 6% | 70% | 64% | 1% | <1%> | 6% | 13% |
| A(H3N2) | 52% | 58% | 3% | 13% | 96% | >99% | 17% | 23% |
| B/Victoria | 1% | 1% | 24% | 18% | 1% | <1%> | <1%> | <1%> |
| B/Yamagata | 38% | 35% | 2% | 6% | 2% | <1%> | 77% | 64% |
Virological analyses
A representative as possible selection (in terms of geographical locations, collection time periods, and types/subtypes/lineage types) of the influenza-positive samples from laboratories and from the sentinel surveillance programme are further analysed for genotypic features by sequencing and for phenotypic sensitivity to neuraminidase (NA) inhibitors. Swedish laboratories are also asked to send influenza-positive samples from severely ill or deceased patients, patients with vaccine break-through infections, and patients who do not respond to antiviral treatment.
The main sequencing technology used is NGS (Next Generation Sequencing), which allows sequencing of all known influenza A subtypes and both influenza B lineage types. The haemagglutinin (HA) gene is characterised with respect to genetic vaccine similarity, clade affiliation and changes in receptor affinity (lung receptors versus upper respiratory tract receptors). In addition, the HA target sequences for the subtype/lineage-specific real-time PCR systems used for detection of influenza in clinical samples are analysed for sequence mismatches compared to the real-time PCR primers and probes. The NA gene is analysed with respect to reduced or highly reduced inhibition by NA inhibitors. In addition to NGS, Sanger sequencing of the NA gene is used for more urgent requests of genotypic antiviral resistance testing. Two aspects of the matrix protein (M) gene are analysed by sequencing, and the M2 gene of influenza A is analysed with respect to resistance to amantadine while the M target sequences of both influenza A and B of the real-time PCR systems are analysed for sequence mismatches. The genes for non-structural protein 1 (NS1) and polymerase basic protein 2 (PB2) are analysed for mutations associated with changes in virulence.
Phenotypic analysis of sensitivity to the NA inhibitors oseltamivir (Tamiflu®/Ebilfumin®) and zanamivir (Relenza®) is performed with the NAI assay, which requires viruses isolated on cell culture.
A representative selection of the isolated virus samples is sent to the WHO CC in London for antigenic characterisation and for phenotypic analysis of sensitivity to NA inhibitors by NAI assay.
All characterisation data are reported to TESSy and to the Global Initiative on Sharing All Influenza Data (GISAID).
Characterisation of viruses
Table 15 summarises the number of sequenced genes per subtype/lineage for the 2017–2018 season.
| H | N | M | NS | PB2 | PB1 | NP | PA | |
|---|---|---|---|---|---|---|---|---|
| A(H3N2) | 104 | 104 | 109 | 105 | 98 | 95 | 103 | 99 |
| A(H1N1)pdm09 | 48 | 49 | 52 | 52 | 48 | 45 | 50 | 48 |
| B/Yamagata | 123 | 122 | 118 | 114 | 112 | 113 | 108 | 106 |
| B/Victoria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HA - Haemagglutinin. NA - Neuraminidase. M - Matrix protein. NS - Non-structural protein. PB2 - Polymerase basic protein 2. PB1 - polymerase basic protein 1. NP - Nucleoprotein. PA - Polymerase acidic protein. | ||||||||
Characterisation of influenza A(H3N2)
The HA gene of 104 A(H3N2) viruses was further analysed by sequencing. Of these viruses, 63 (61%) belonged to clade 3C.2a, 38 (36 %) belonged to subclade 3C.2a1, and 3 (3%) belonged to clade 3C.3a (see phylogenetic tree in Appendix 1). A similar distribution between these clades and subclades was also seen among the viruses circulating in Europe this season (11). Circulating viruses have been shown to be antigenically similar to both the cell-propagated vaccine virus A/Hong Kong/4801/2014 (in clade 3C.2a) recommended for the northern hemisphere season 2017–2018 and the cell-propagated vaccine virus A/Singapore/INFIMH-16-0019/2016 (in subclade 3C.2a1) recommended for the northern hemisphere season 2018–2019, while the antigenic similarity to egg-propagated A/HongKong4801/2014 was low. Analysed viruses in Europe showed greater similarity to the egg-propagated strain A/Singapore/INFIMH-16-0019/2016 compared to other recent egg-propagated A(H3N2)-viruses (5). In Sweden, five viruses from vaccinated individuals were further analysed by sequencing. All five were sampled from individuals that were 65 years or older. Three of the viruses belonged to clade 3C.2a and two belonged to subclade 3C.2a1.
The NA gene of 104 A(H3N2) viruses was analysed, and none of them harboured any mutations known to be associated with reduced or highly reduced inhibition to oseltamivir or zanamivir. In addition, 19 A(H3N2) viruses were analysed phenotypically with respect to sensitivity to the NA inhibitors oseltamivir and zanamivir. All were shown to be sensitive to both inhibitors. Sixteen of these viruses were also analysed by the WHO CC with concordant results. The WHO CC also analysed three additional viruses from Sweden that were shown to be sensitive to both inhibitors. In the European surveillance system, three viruses with reduced inhibition to NA inhibitors were detected during season 2017–2018 (11).
All but one of the 109 viruses from Sweden for which the M2 gene was sequenced were resistant to amantadine due to the substitution S31N. Three of these also carried the V27I mutation that also confers resistance to amantadine. The NS gene of 105 analysed viruses and the PB2 gene of 98 analysed viruses did not contain any mutations known to be associated with increased virulence. Eleven of these viruses were collected from severe cases requiring intensive care or ECMO treatment. Both NS and PB2 could be analysed in nine of the cases, while only NS could be analysed in two of the cases.
Characterisation of influenza A(H1N1)pdm09
All 48 A(H1N1)pdm09 viruses for which the HA gene was sequenced belonged to subclade 6B.1 (see phylogenetic tree for influenza A(H1N1)pdm09 in Appendix 2), as did all viruses reported in the European surveillance system in 2017–2018 (11). The vast majority of circulating viruses have been shown to be antigenically similar to the egg-propagated vaccine virus recommended for the northern hemisphere in both 2017–2018 and 2018-2019 (A/Michigan/45/2015), which also belongs to clade 6B.1 (5). One virus from a vaccinated individual (aged 38 years and without any known underlying immunosuppression) was sequenced by the Public Health Agency.
Of the 49 viruses where the NA gene was sequenced, 48 did not have any mutations known to be associated with reduced or highly reduced inhibition to oseltamivir or zanamivir. One sample from a transplant patient, and thus likely treated with NA inhibitor, had both viruses with the H275Y mutation and non-mutated virus. The H275Y mutation causes highly reduced inhibition to oseltamivir but not to zanamivir. In addition, 72 viruses were analysed exclusively for the H275Y mutation and were all shown to be non-mutated (H275). Five A(H1N1)pdm09 viruses were also analysed phenotypically with respect to sensitivity to the NA inhibitors oseltamivir and zanamivir by both the Public Health Agency of Sweden and the WHO CC, and all five were shown to be sensitive to both inhibitors. In the European surveillance system, 11 viruses with the H275Y mutation were reported (among 566 analysed). No mutations conferring reduced or highly reduced inhibition were reported in the remaining 545 viruses (11).
All 52 analysed Swedish viruses were resistant to amantadine due to the S31N substitution in the M2 gene. No amino acid substitutions known to be associated with increased virulence were seen in position 222 of HA subunit 1 (48 analysed viruses), the NS1 gene (52 analysed viruses), or the PB2 gene (48 analysed viruses).
Characterisation of B/Yamagata
Analysis of 123 B/Yamagata-like viruses by sequencing of the HA gene showed that these belonged to genetic clade 3 (see phylogenetic tree in Appendix 3). This was also the dominant clade among the viruses reported in the European surveillance this season (11). The majority of circulating B/Yamagata viruses have reacted well against ferret antisera raised against both cell-propagated and egg-propagated B/Phuket/3073/2013, the virus that is included in the quadrivalent vaccine but not in the trivalent vaccine for the northern hemisphere in the 2017–2018 season. This virus is also recommended for the quadrivalent vaccine for the northern hemisphere season 2018–2019 (5). As noted above, the B/Victoria virus included in the trivalent vaccine likely confers some cross-protection to B/Yamagata viruses (12). Among the 123 Swedish B/Yamagata viruses for which HA was sequenced, 10 originated from vaccinated individuals; although this information is missing, these patients were most likely vaccinated with the trivalent vaccine. Of these, six were aged 65 years or older, while the remaining four ranged in age from 8 to 63, including one individual with underlying immunosuppression.
None of the 122 B/Yamagata viruses for which the NA gene was sequenced had any mutations known to be associated with reduced or highly reduced inhibition to oseltamivir or zanamivir. In addition, six B/Yamagata viruses were analysed phenotypically with respect to sensitivity to NA inhibitors, and all were sensitive to both inhibitors. Five of these were also analysed by the WHO CC with concordant results. The WHO CC also analysed one additional virus from Sweden that was shown to be sensitive to both inhibitors. In the European surveillance system, five influenza B viruses with reduced inhibition to neuraminidase inhibitors were detected during 2017–2018 (11).
Characterisation of B/Victoria
None of the B/Victoria-positive samples (including one double infection with B/Yamagata) that were sent in for further characterisation had enough viral copies for sequencing or isolation, which is required for an NAI assay. In the European surveillance system, 55 percent of 152 viruses belonged to genetic clade 1A, while 45 percent belonged to clade 1Adel162-163 (11). The majority of circulating viruses were antigenically related to the cell-propagated vaccine virus included in the trivalent vaccine for season 2017–2018 (B/Brisbane/60/2008), which belongs to clade 1A. However, viruses with a deletion of HA1 amino acids 162–163 have reacted poorly to B/Brisbane/60/2008 in antigenic analyses. Therefore, the WHO has recommended that the 1Adel162-163 virus B/Colorado/0672017 should be included in the trivalent and quadrivalent vaccines for the northern hemisphere in the 2018–2019 season (5).
Virus isolation on cell culture
The majority of the samples selected for isolation are collected from other laboratories. The quality of the samples differs depending on, for example, the type of specimen, the time since sampling, and the storage and shipping conditions. Sixty of the collected samples with Ct ≤ 30 were cultured on MDCK cells. Seven samples were excluded due to contamination with bacteria or fungi, and 64 percent of the remaining samples tested positive for influenza.
During the 2017–2018 season, 35 virus isolates and 24 clinical samples were shipped to the WHO CC for further characterisation (one shipment in January and one in June).
Vaccination coverage
Data on vaccination coverage among persons 65 years of age and older have been gathered by Sweden’s 21 county medical officers for their respective county councils since 2003. The Public Health Agency took over this task in 2014. Various methods for estimation have been and are used in different counties, including the use of vaccination registries, the number of vaccine doses given or distributed, sentinel reports on vaccination coverage, surveys among general practitioners, or patient record data. These methodological differences result in coverage estimates of varying quality and precision. Although the methods vary between counties, the methods within most counties have been roughly the same for the past several years. An estimate of the vaccination coverage in other age groups is included, using data from a subset of county councils where registry data by age group are available throughout the season as well as annually. Data for 2017–2018 come from Gävleborg, Jämtland Härjedalen, Jönköping, Kalmar, Kronoberg, Norrbotten, Skåne, Stockholm, Värmland, Västernorrland, Västmanland, Västra Götaland, and Östergötland.
Coverage among those 65 years of age and older
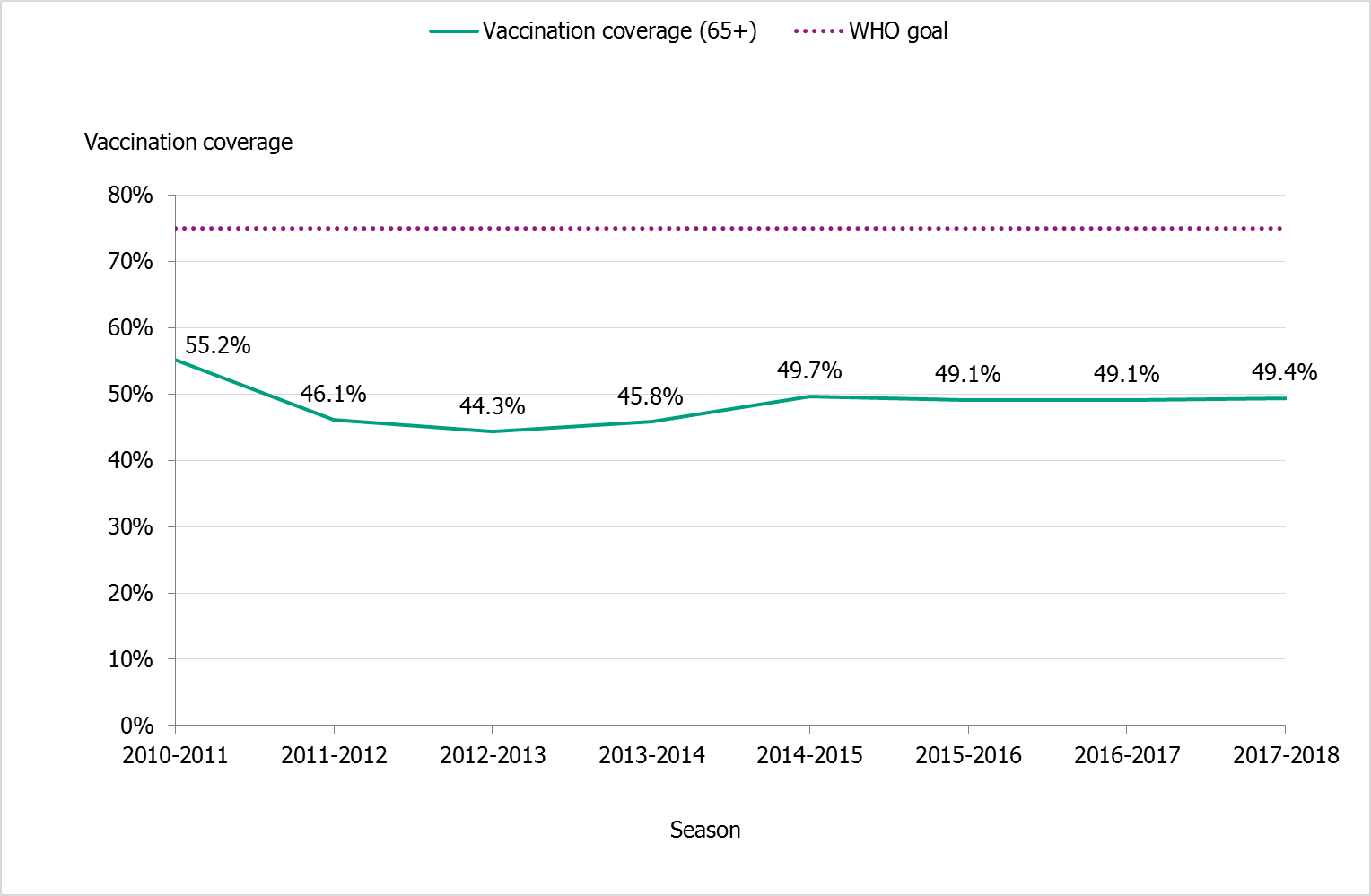
The national vaccination rate among those aged 65 and over was 49.4 percent in 2017–2018 (see Figure 14). Vaccination coverage in this age group has remained steady at just under 50 percent for the past several years. In total, approximately 960,000 people aged 65 and older were vaccinated. Coverage was highest among people aged 75 and older (55 percent, see Table 16), which is encouraging because the risk of severe influenza increases with age. However, none of the age groups’ coverage rates approached the WHO target of 75 percent.
Figure 14. Vaccination coverage among those aged 65 and older in Sweden, 2010–2011 to 2017–2018.
Regional differences in vaccination coverage
Comparisons among counties/regions are difficult because estimates are based on different methods. There is uncertainty associated with each value, but the figure below gives a picture of the current vaccination coverage rates for the age group 65 years and older throughout Sweden (see Figure 15). For more regional data and collection methods, see the Swedish-language summary of the season published in June 2018 (13).
Figure 15. Estimated proportion of vaccinated persons aged 65 and older per county council in Sweden for seasons 2016–2017 and 2017–2018.
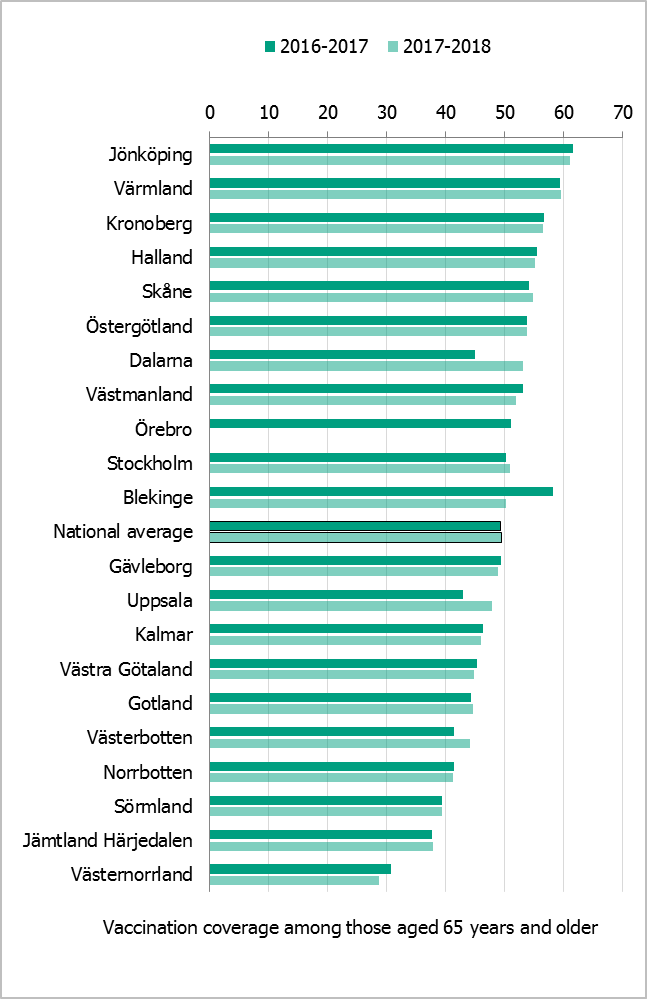
*Notes: Different estimation methods were used in each county, which makes comparison difficult. Percentages are based on the population of the county on December 31 of each year (Source: Statistics Sweden.) In Gävleborg, vaccination registration is tied to financial incentives. Data from Jämtland Härjedalen and Västernorrland do not include doses given in long-term care facilities, etc., which underestimates the coverage rate. Three coverage estimates for 2016–2017 were available for Skåne Region using data from a survey (56%), billing (54.4%), and the vaccine registry (45.5%). For 2017–2018, there are two estimates for Skåne Region – from billing (54.7%) and from the vaccine registry (45.3%). In this report, we use the coverage rate based on billing data. Data from Örebro for the 2017–2018 season were not yet available at the time of writing.
Vaccination coverage in medical risk groups
It is difficult to estimate vaccination coverage among the medical risk groups because these groups are hard to define and because data are often missing. Approximately 5–10 percent of the population under 65 years of age belong to a medical risk group.
Twelve county councils (see note under Table 16) have reported the number of persons vaccinated under 65 years of age, although risk group status is often unknown. An analysis of these data shows that, once again, only about 2 percent of those under 65 years of age were vaccinated during the season (see Table 16 for breakdown by age group). The coverage in most age groups is similar to that seen in the previous seasons and indicates that many of those who could benefit the most from vaccination are not reached. However, vaccination coverage increased from 4 percent to 11 percent in the age group 40–64 years, although we do not know what proportion of these vaccinations were given to persons with a medical risk for severe influenza.
| Age group | 0–17 years | 18–39 years | 40–64 years | 65–74 years | 75–84 years | 85+ years |
|---|---|---|---|---|---|---|
| Percentage vaccinated | 0.2% | 1.3% | 11.0% | 41.3% | 55.3% | 55.8% |
| Data for 2017–2018 come from Gävleborg, Jämtland Härjedalen, Jönköping, Kalmar, Kronoberg, Norrbotten, Skåne, Stockholm, Värmland, Västernorrland, Västmanland, and Västra Götaland. | ||||||
Syndromic surveillance of community disease burden
Two systems of syndromic surveillance were used this season – Webbsök (Web search) and telephone calls to the medical advice line 1177 Vårdguiden. Webbsök is an automated system established in 2008 that uses a statistical model and completely anonymous data from a medical advice website to estimate the development of sentinel influenza-like illness (ILI) incidence. Data are received daily and collated weekly. The results are published on the web every Monday morning during the influenza season in the form of a graph, which is three days ahead of the publication of the weekly influenza bulletin.
In collaboration with the medical telephone advice line 1177 Vårdguiden, the Public Health Agency receives aggregated weekly data on calls from a system called Hälsoläge. The age group and main reason for calling are registered for all callers. The reported data include the number of calls related to cough, fever, and sore throat. The proportion of calls related to fever in children has been found to be a good indicator of influenza activity in the community.
Web search data (Webbsök)
Webbsök was the first system to cross the epidemic threshold during the 2017–2018 season and indicated that the influenza season started in week 43, 2017. From week 27, 2017, to week 26, 2018, a total of 394,960 queries related to influenza were submitted to the 1177.se search engine, which was 4.7 percent of all searches. As a percentage of searches, this was higher than the previous season, when approximately 450,000 searches made up 4.3 percent of all searches.
According to Webbsök, the 2017–2018 influenza season lasted for 25 weeks, from week 43, 2017, to week 15, 2018 (Figure 16). During five of these weeks (weeks 5–9), Webbsök showed a high level of influenza activity, of which week 7 to week 8 was at a very high level. This is higher than the 2016–2017 season when influenza activity lasted for 19 weeks and reached only a medium level for 4 weeks. The seasonal pattern this season corresponds largely to that seen in laboratory-based surveillance that indicated that the influenza season started in week 49, 2017, and peaked in week 7, 2018 (Figure 17). By providing data on Monday mornings, Webbsök provides an early indication of the activity in the previous week.
Figure 16. Webbsök’s estimated proportion of the population with ILI per week, 2015–2018. Start and end points of the epidemic are marked with black dots.
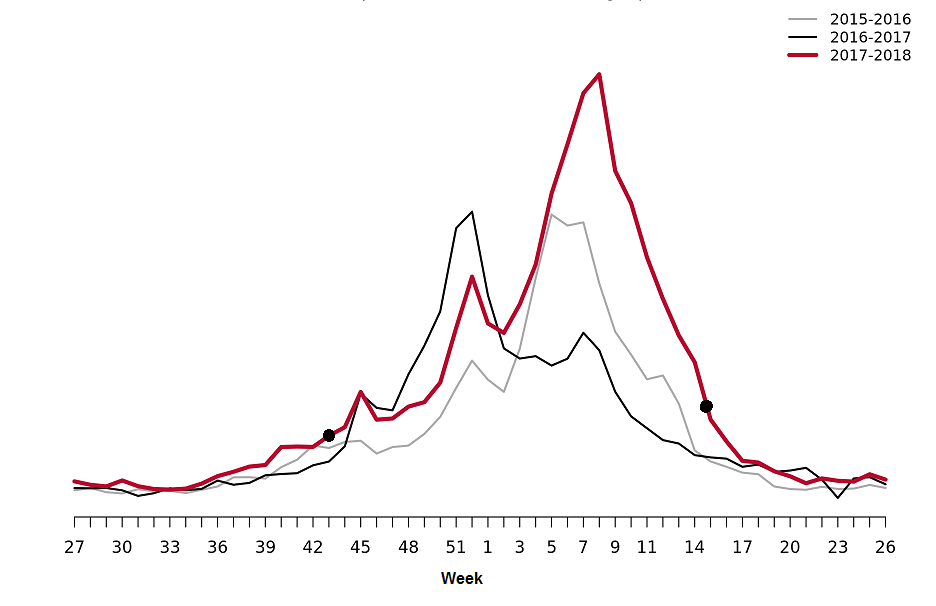
Figure 17. Webbsök’s estimated proportion of persons with ILI (incidence per 100,000 population) and the number of laboratory-confirmed cases, 2017–2018.
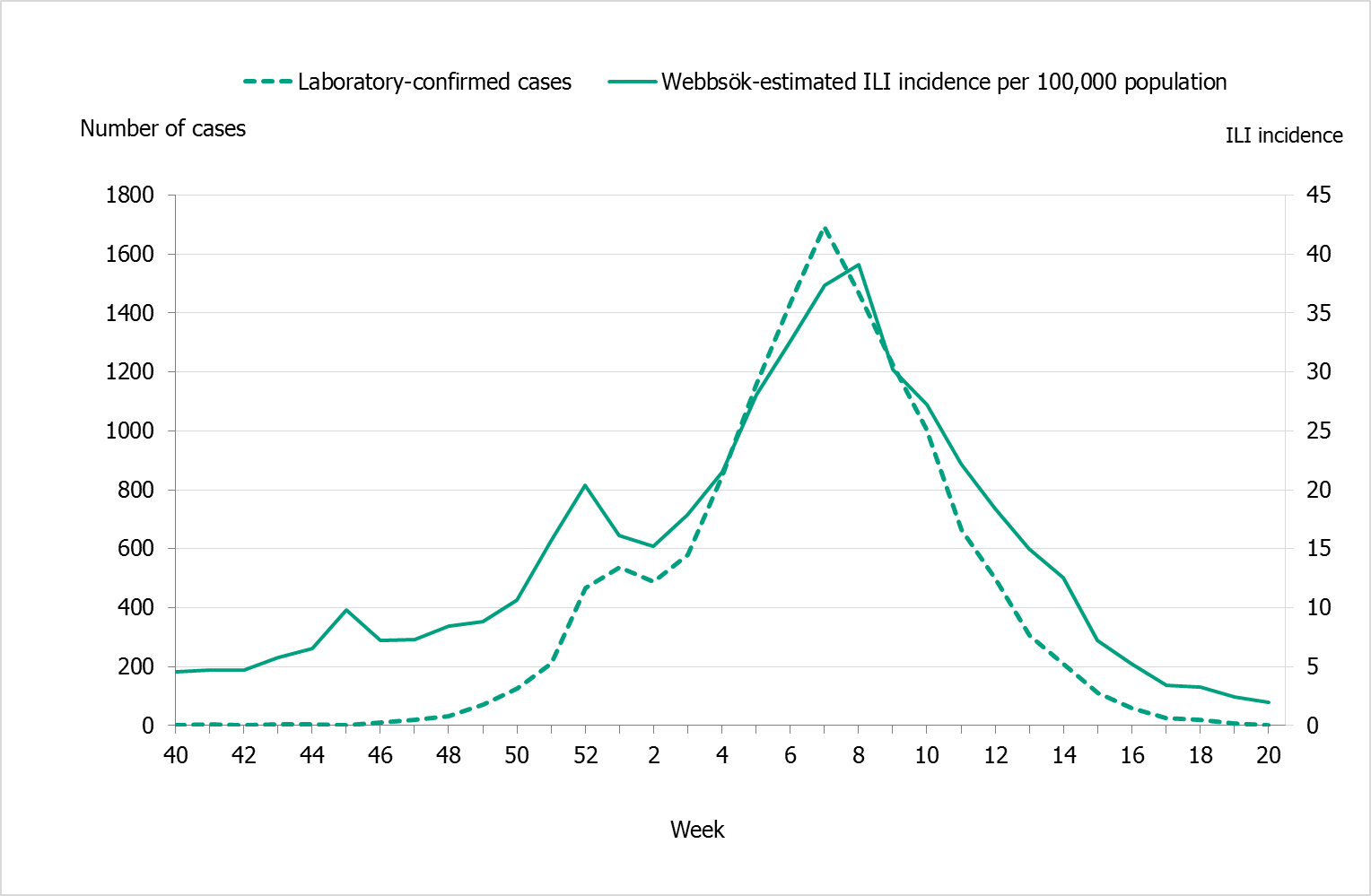
Telephone advice line data (1177 Vårdguiden)
The number of calls to 1177 Vårdguiden regarding fever in children exceeded the epidemic threshold for the 2017–2018 season in week 52, peaked in week 7, and ended in week 17. The number of calls was at a low level for most of the season, but reached a medium level during weeks 4–9, 2018.
An average of 5.4 percent of the calls throughout the season (weeks 40–20) were regarding children with fever (Figure 18), which was higher than the previous season when this figure was 4.9 percent. The highest number (4,039) and percentage of calls (8.6 percent) was registered during week 7, 2018. This peak corresponded to the peak in the laboratory-based surveillance.
A noticeable peak in calls is often seen around the Christmas holidays every year, followed by a drop. The reason for this pattern might be decreased access to face-to-face health care services during the holidays, leading to an increase in telephone consultations.
Figure 18. Percentage of telephone calls regarding fever in children received by the medical advice line 1177 Vårdguiden for the past three seasons. Start and end points of the epidemic are marked with black dots.
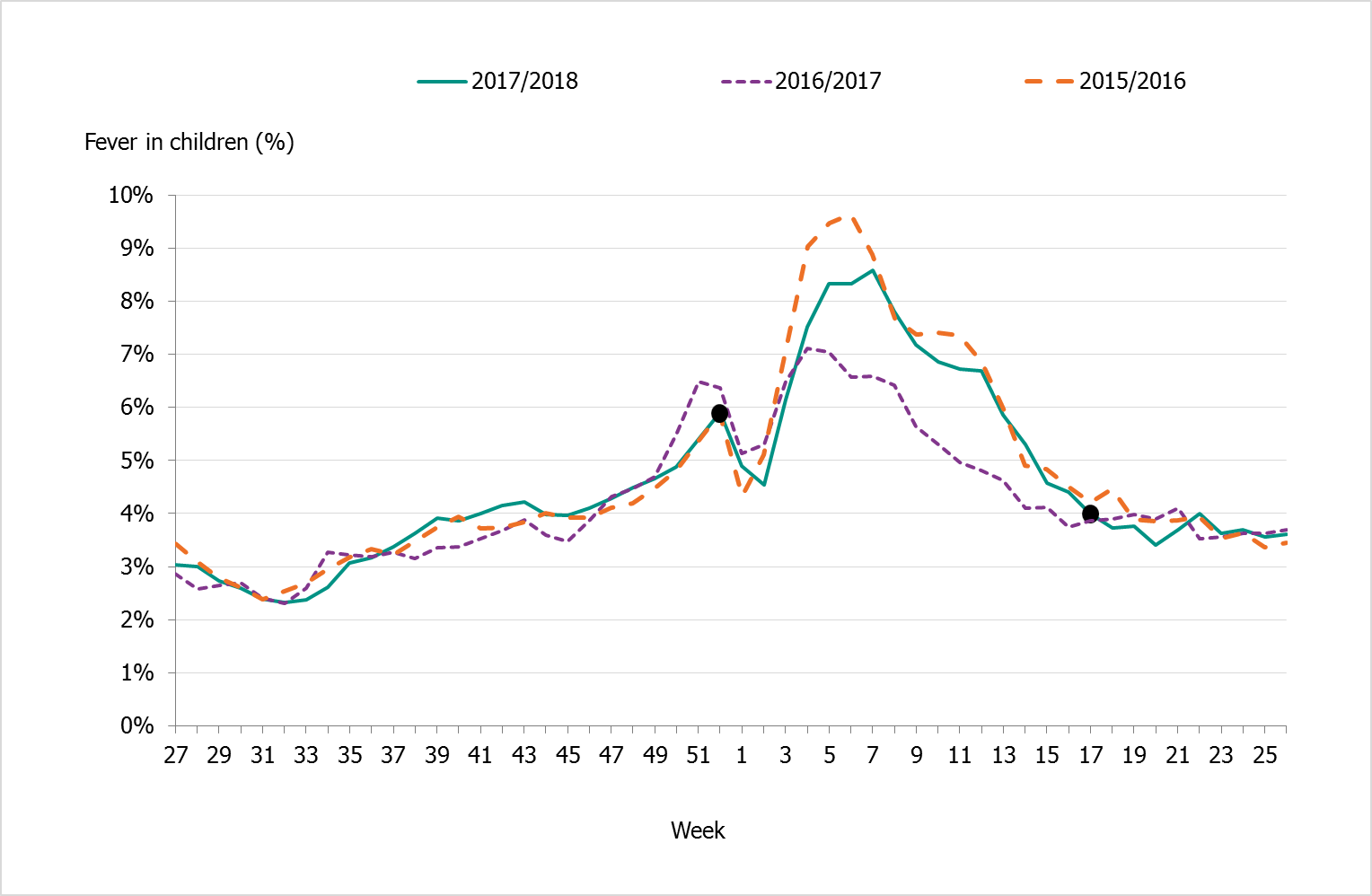
Quality assurance
Different external quality assurance programmes are used to ensure the quality of the diagnostics done in Sweden and of the analyses done by the Public Health Agency. Surveillance is dependent on standardised virological methods.
At the Public Health Agency, one-step real-time RT-PCR assays are used detect influenza A and B, to subtype the influenza A-positive samples, and to discriminate between the two influenza B lineages. These assays have also been evaluated and implemented for avian influenza diagnostics. They are sensitive, rapid, and can easily be scaled up if necessary. The Public Health Agency continuously monitors the genomic sequences of circulating influenza strains to which the PCR assays are directed in order to detect mutations that could affect their sensitivity. The Public Health Agency also performs regular validation of each assay twice a year, both ahead of the influenza season and during the peak. The PCR protocols are shared with the other laboratories in Sweden, and the laboratories that use these PCR systems are encouraged to send all samples with deviating results to the agency for sequence analysis.
Yearly, the Public Health Agency produces a panel PCR for the Swedish laboratories on behalf of the External Quality Assessment for Clinical Laboratory Investigations (EQUALIS). This allows the laboratories to measure the analytic sensitivity and specificity of their method. The majority of the laboratories performing diagnostics for influenza use commercial rapid PCR kits. In the beginning of the season, a questionnaire is sent to all laboratories with a question concerning methods used. Kits used by two or more laboratories are selected for extra quality controls during the season in order to ensure that circulating influenza strains are detected with these assays.
To ensure that the analyses performed at the Public Health Agency are correct, the agency participates in different external quality assurance programmes.
National quality assurance programme for influenza PCR
In September 2017, the Public Health Agency produced a new PCR panel for the Swedish laboratories, which was distributed by EQUALIS. Twenty laboratories participated in the panel, and 14 of these reported 10/10 correct results (Figure 19). The remaining six laboratories reported 9/10 correct results. The six laboratories did not detect influenza A in the sample with the lowest viral load.
Figure 19. Results of the Swedish External Quality Assessment panel 2017
Control of commercial rapid PCR-kits
During the 2017–2018 season, an increasing number of laboratories used commercial rapid PCR kits for influenza diagnostics. Results of a questionnaire sent to all 25 microbiological laboratories in Sweden showed that 21 laboratories used a commercial PCR assay, one laboratory used an in-house real-time PCR assay, and three laboratories combined both commercial and in-house assays. The use of rapid PCR kits has increased the availability of diagnostics, and this season 13 laboratories offered diagnostic service around the clock, seven days a week.
Ten different kits were tested this season:
- Simplexa™ Influenza A/B & RSV Kit (Focus Diagnostics)
- Cobas® Liat® Influenza A/B & RSV (Roche)
- Xpert® Influenza (Cepheid)
- Xpert® Flu/RSV XC (Cepheid)
- Xpert® Xpress Influenza (Cepheid)
- Alere™ Influenza A & B Test (Abbott)
- Allplex, panel 1, 2, and 3 (Seegene)
- LightMix® Modular A, B, RSV (Roche)
- FILMARRAY® Respiratory Panel (Biomérieux)
- FTD Respiratory pathogens 33 (Fast Track Diagnostics)
The laboratories received a number of samples on two occasions – at the beginning and during the peak of the season. A total of eight positive samples (diluted isolates) were tested with the different kits, and all gave 100% correct results with one exception. The FilmArray respiratory panel version 1 detected all samples in the two panels but gave the result Influenza A ”Equivocal” for one sample (not subtypeable), even when the analysis was repeated. When using FilmArray respiratory panel version 2, the correct subtype, A(H3), was detected. The results were shown on the Public Health Agency website so that other laboratories using the same kits could have knowledge of the results.
External quality assurance programmes
The Public Health Agency participated in two external quality assurance programmes during 2017–2018.
Annual WHO External Quality Assessment panel for influenza (no. 16) 2017
The real-time PCR result was 10/10 correctly typed and subtyped. Phenotypic NAI susceptibility (fluorescence-based assay with MUNANA as the substrate) and genotypic NAI susceptibility (performed with NGS) was correctly reported for the two samples included in the panel.
INFRNA panel from Quality Control for Molecular Diagnostics (QCMD) 2017
The real-time PCR result was 10/10 samples correctly typed (A or B) and 8/10 correctly subtyped. The two samples that could not be subtyped were both pre-2009 seasonal A(H1N1), and there is no teal-time PCR system in place at our laboratory at present for detecting that subtype However, these samples could be subtyped by NGS.
References
- Public Health Agency of Sweden. Publikationer [Publications]. [Internet]. Stockholm: Public Health Agency of Sweden; 2018 [cited 2018-08-08] Available from: http://www.folkhalsomyndigheten.se/publicerat-material/publikationer/
- Public Health Agency of Sweden, Aktuell influensarapport [Current Weekly Report]. [Internet]. Stockholm: Public Health Agency of Sweden; 2018 [cited 2018-08-08] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistikdatabaser-och-visualisering/sjukdomsstatistik/influensa-veckorapporter/aktuell-influensarapport/
- Public Health Agency of Sweden, Arkiv 2017/2018 [Influenza Weekly Report Archive, 2017–2018]. [Internet]. Stockholm: Public Health Agency of Sweden; 2018 [cited 2018-08-08] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistikdatabaser-och-visualisering/sjukdomsstatistik/influensa-veckorapporter/arkiv-20172018/
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2017- 2018 northern hemisphere influenza season [Internet]. Geneva: World Health Organization; 2017. [published 2017-03-2, cited 2018-08-08]. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/201703_recommendation.pdf?ua=1
- World Health Organization. WHO, Recommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season [Internet]. Geneva: World Health Organization; 2017. [published 2018-02-22, cited 2018-08-08]. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/2018_19_north/en/
- Public Health Agency of Sweden, Rekommendationer om influensavaccination till riskgrupper [Recommendations for influenza vaccination of risk groups]. Stockholm: Public Health Agency of Sweden; 2018 [cited 2018-08-08] Available from: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/r/rekommendationer-om-influensavaccination-till-riskgrupper/
- Public Health Agency of Sweden, Vaccinationsplan vid influensapandemi [Pandemic Vaccination Plan]. Stockholm: Public Health Agency of Sweden; 2017 [cited 2018-08-08] Available from: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/v/vaccinationsplan-vid-influensapandemi/
- Public Health Agency of Sweden, Pandemiberedskap [Pandemic preparedness]. [Internet] Stockholm: Public Health Agency of Sweden; 2018 [updated 2018-05-25, cited 2018-08-08] Available from: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/krisberedskap/pandemiberedskap/
- EuroMoMo Project, Mortality monitoring in Europe [Internet]. Danmark: EuroMoMo Project; 2018 [cited 2018-08-08]. Available from: http://www.euromomo.eu/
- Rondy M, Kissling E, Emborg H D, Gherasim A, Pebody R, Trebbien R, et al. Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies, Euro Surveill, 2018 Mar;23(9). Available from: https://www.ncbi.nlm.nih.gov/pubmed/29510782
- European Centres for Disease Control and Prevention (ECDC), World Health Organisation European Office (WHO), Influenza News Europe, week 20, 2018 [Internet]. Stockholm and Denmark: ECDC and WHO; 2018 [cited 2018-08-08]. Available from: http://flunewseurope.org/Archives
- World Health Organisation. Questions and Answers Recommended composition of influenza virus vaccines for use in the southern hemisphere 2018 influenza season, [Internet]. Geneva: World Health Organisation; 2018. [updated 2018-09-28, cited 2018-08-08] Available from: http://www.who.int/influenza/vaccines/virus/recommendations/201709_qanda_recommendation.pdf?ua=1
- Public Health Agency of Sweden, Sammanfattande rapport för säsongen 2017–2018 [Summary report for the 2017–2018 season]. [Internet] Stockholm: Public Health Agency of Sweden; 2018 [updated 2018-07-06, cited 2018-08-22] Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistikdatabaser-och-visualisering/sjukdomsstatistik/influensa-veckorapporter/arkiv-20172018/
